当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Escape sites from the endo-lysosomal trafficking route manipulate exocytosis of nanoparticles in polar epithelium
Biomaterials Science ( IF 6.6 ) Pub Date : 2024-04-02 , DOI: 10.1039/d4bm00174e Lingling Wang , Yuting Li , Xi Liu , Liyun Xing , Ruinan Wu , Yuan Huang
Biomaterials Science ( IF 6.6 ) Pub Date : 2024-04-02 , DOI: 10.1039/d4bm00174e Lingling Wang , Yuting Li , Xi Liu , Liyun Xing , Ruinan Wu , Yuan Huang
|
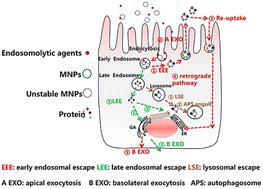
The endo–lysosomal pathway is a major barrier for the trans-epithelial transport of nanoparticles (NPs), but escape strategies could facilitate trans-epithelial delivery. Based on the polarization properties of the epithelium, different escape compartments may result in different exocytosis fates of NPs and further affect the delivery efficiency. Therefore, optimizing the escape sites is critical for trans-epithelial delivery. Here, commonly used PEG-coated-poly(lactic-co-glycolic acid) (PLGA)-based nanoparticles were fabricated as model nanoparticles (MNPs) and the intestinal epithelium was chosen as the polarized epithelium. The MNPs were incubated with different endosomolytic agents for early endosomal escape, late endosomal escape and lysosomal escape, respectively. According to in vitro and in vivo studies, MNPs escaping from early endosomes and late endosomes exhibited stronger capacity for trans-epithelial transport than those escaping from lysosomes. By further probing into the mechanism, we surprisingly found that although MNPs escaping from early endosomes quickly egressed from the apical side of epithelia, they were subsequently followed by “reuptake” via caveolae and trafficked through the endoplasmic reticulum–Golgi apparatus (ER/GA) secretory pathway, achieving efficient trans-epithelial transport; MNPs escaping from late endosomes, which were located near the nucleus, were prone to enter the ER/GA for efficient basolateral exocytosis. However, MNPs escaping from lysosomes were detained within cells by autophagosomes. Collectively, our research suggested that early endosomes and late endosomes were ideal escape sites for trans-epithelial delivery.
中文翻译:

内溶酶体运输途径的逃逸位点操纵极上皮中纳米颗粒的胞吐作用
内溶酶体途径是纳米颗粒(NP)跨上皮运输的主要障碍,但逃逸策略可以促进跨上皮运输。基于上皮的极化特性,不同的逃逸室可能导致纳米颗粒不同的胞吐命运,并进一步影响递送效率。因此,优化逃逸位点对于跨上皮递送至关重要。在这里,常用的基于 PEG 涂层的聚乳酸乙醇酸( PLGA) 的纳米颗粒被制造为模型纳米颗粒 (MNP),并选择肠上皮作为极化上皮。将MNP与不同的内体溶解剂一起孵育,分别进行早期内体逃逸、晚期内体逃逸和溶酶体逃逸。根据体外和体内研究,从早期内体和晚期内体逃逸的MNP比从溶酶体逃逸的MNP表现出更强的跨上皮运输能力。通过进一步探究其机制,我们惊讶地发现,虽然从早期内体逃逸的MNP很快从上皮细胞的顶端流出,但它们随后通过小凹“再摄取”并通过内质网-高尔基体(ER/GA)运输分泌途径,实现高效的跨上皮运输;从位于细胞核附近的晚期内体中逃逸的 MNP 易于进入 ER/GA 进行有效的基底外侧胞吐作用。然而,从溶酶体逃逸的 MNP 被自噬体保留在细胞内。总的来说,我们的研究表明早期内体和晚期内体是跨上皮递送的理想逃逸位点。
更新日期:2024-04-02
中文翻译:

内溶酶体运输途径的逃逸位点操纵极上皮中纳米颗粒的胞吐作用
内溶酶体途径是纳米颗粒(NP)跨上皮运输的主要障碍,但逃逸策略可以促进跨上皮运输。基于上皮的极化特性,不同的逃逸室可能导致纳米颗粒不同的胞吐命运,并进一步影响递送效率。因此,优化逃逸位点对于跨上皮递送至关重要。在这里,常用的基于 PEG 涂层的聚乳酸乙醇酸( PLGA) 的纳米颗粒被制造为模型纳米颗粒 (MNP),并选择肠上皮作为极化上皮。将MNP与不同的内体溶解剂一起孵育,分别进行早期内体逃逸、晚期内体逃逸和溶酶体逃逸。根据体外和体内研究,从早期内体和晚期内体逃逸的MNP比从溶酶体逃逸的MNP表现出更强的跨上皮运输能力。通过进一步探究其机制,我们惊讶地发现,虽然从早期内体逃逸的MNP很快从上皮细胞的顶端流出,但它们随后通过小凹“再摄取”并通过内质网-高尔基体(ER/GA)运输分泌途径,实现高效的跨上皮运输;从位于细胞核附近的晚期内体中逃逸的 MNP 易于进入 ER/GA 进行有效的基底外侧胞吐作用。然而,从溶酶体逃逸的 MNP 被自噬体保留在细胞内。总的来说,我们的研究表明早期内体和晚期内体是跨上皮递送的理想逃逸位点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号