Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-04-02 , DOI: 10.3762/bjoc.20.62 Jan Bartáček , Karel Chlumský , Jan Mrkvička , Lucie Paloušová , Miloš Sedlák , Pavel Drabina
Abstract
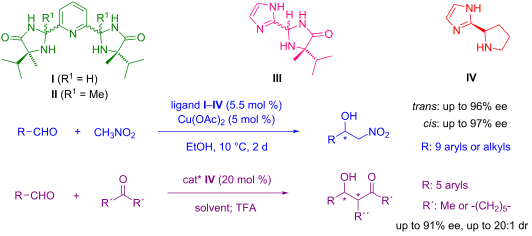
The new chiral ligands I–III based on derivatives of imidazolidin-4-one were synthesised and characterised. The catalytic activity and enantioselectivity of their corresponding copper(II) complexes were studied in asymmetric Henry reactions. It was found that the enantioselectivity of these catalysts is overall very high and depends on the relative configuration of the ligand used; cis-configuration of ligand affords the nitroaldols with major enantiomer S- (up to 97% ee), whereas the application of ligands with trans-configuration led to nitroaldols with major R-enantiomer (up to 96% ee). The “proline-type” ligand IV was also tested in asymmetric aldol reactions. Under the optimised reaction conditions, aldol products with enantioselectivities of up to 91% ee were obtained.
Beilstein J. Org. Chem. 2024, 20, 684–691. doi:10.3762/bjoc.20.62
中文翻译:

基于咪唑烷-4-酮衍生物的新型手性配体的对映选择性评价
摘要
合成并表征了基于咪唑啉-4-酮衍生物的新型手性配体I-III 。在不对称亨利反应中研究了相应铜(II)配合物的催化活性和对映选择性。研究发现,这些催化剂的对映选择性总体上非常高,并且取决于所用配体的相对构型;配体的顺式构型提供了主要对映体S-的硝基羟醛(高达97% ee),而具有反式构型的配体的应用导致硝基羟醛具有主要对映体R-对映体(高达96% ee)。 “脯氨酸型”配体IV也在不对称羟醛反应中进行了测试。在优化的反应条件下,得到对映选择性高达91% ee的羟醛产物。

贝尔斯坦 J. 组织。化学。 2024, 20, 684–691。 doi:10.3762/bjoc.20.62



























 京公网安备 11010802027423号
京公网安备 11010802027423号