Transgenic Research ( IF 3 ) Pub Date : 2024-04-04 , DOI: 10.1007/s11248-024-00375-z H. M. Gruchow , P. Opdensteinen , J. F. Buyel
|
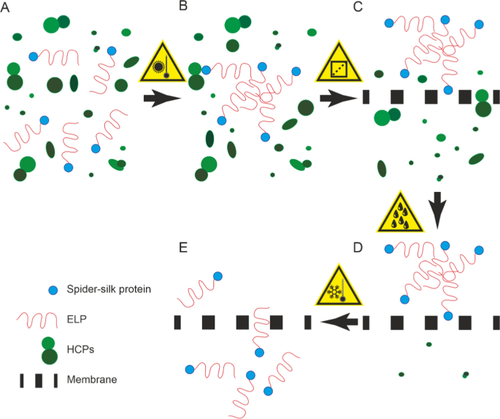
Plants can produce complex pharmaceutical and technical proteins. Spider silk proteins are one example of the latter and can be used, for example, as compounds for high-performance textiles or wound dressings. If genetically fused to elastin-like polypeptides (ELPs), the silk proteins can be reversibly precipitated from clarified plant extracts at moderate temperatures of ~ 30 °C together with salt concentrations > 1.5 M, which simplifies purification and thus reduces costs. However, the technologies developed around this mechanism rely on a repeated cycling between soluble and aggregated state to remove plant host cell impurities, which increase process time and buffer consumption. Additionally, ELPs are difficult to detect using conventional staining methods, which hinders the analysis of unit operation performance and process development. Here, we have first developed a surface plasmon resonance (SPR) spectroscopy-based assay to quantity ELP fusion proteins. Then we tested different filters to prepare clarified plant extract with > 50% recovery of spider silk ELP fusion proteins. Finally, we established a membrane-based purification method that does not require cycling between soluble and aggregated ELP state but operates similar to an ultrafiltration/diafiltration device. Using a data-driven design of experiments (DoE) approach to characterize the system of reversible ELP precipitation we found that membranes with pore sizes up to 1.2 µm and concentrations of 2–3 M sodium chloride facilitate step a recovery close to 100% and purities of > 90%. The system can thus be useful for the purification of ELP-tagged proteins produced in plants and other hosts.
中文翻译:

基于膜的反向转换纯化有助于从转基因烟草提取物中快速分离各种蜘蛛丝弹性蛋白样多肽融合蛋白
植物可以生产复杂的药物和技术蛋白质。蜘蛛丝蛋白是后者的一个例子,可以用作高性能纺织品或伤口敷料的化合物。如果通过基因方式与类弹性蛋白多肽 (ELP) 融合,丝蛋白可以在 ~ 30 °C 的中等温度和 > 1.5 M 的盐浓度下从澄清的植物提取物中可逆沉淀,从而简化纯化并降低成本。然而,围绕这种机制开发的技术依赖于可溶状态和聚集状态之间的重复循环来去除植物宿主细胞杂质,这增加了处理时间和缓冲液消耗。此外,使用传统的染色方法很难检测ELP,这阻碍了单元操作性能的分析和工艺开发。在这里,我们首先开发了一种基于表面等离子共振 (SPR) 光谱的测定法来定量 ELP 融合蛋白。然后我们测试了不同的过滤器来制备澄清的植物提取物,蜘蛛丝 ELP 融合蛋白的回收率 > 50%。最后,我们建立了一种基于膜的纯化方法,不需要在可溶性和聚集 ELP 状态之间循环,但操作类似于超滤/渗滤装置。使用数据驱动的实验设计 (DoE) 方法来表征可逆 ELP 沉淀系统,我们发现孔径高达 1.2 µm 且氯化钠浓度为 2–3 M 的膜有助于一步实现接近 100% 的回收率和纯度> 90%。因此,该系统可用于纯化植物和其他宿主中产生的 ELP 标签蛋白。



























 京公网安备 11010802027423号
京公网安备 11010802027423号