Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring ionic liquids for the extraction separation of aromatic hydrocarbons from diesel via multiple interaction analyses coupled with experimental evaluation
Fuel ( IF 7.4 ) Pub Date : 2024-04-05 , DOI: 10.1016/j.fuel.2024.131634 Weikang Gao , Chuanlei Liu , Yuxiang Chen , Guanchu Guo , Hao Wang , Fengjing Yang , Hao Jiang , Qiyue Zhao , Qiumin Wu , Benxian Shen , Hui Sun
Fuel ( IF 7.4 ) Pub Date : 2024-04-05 , DOI: 10.1016/j.fuel.2024.131634 Weikang Gao , Chuanlei Liu , Yuxiang Chen , Guanchu Guo , Hao Wang , Fengjing Yang , Hao Jiang , Qiyue Zhao , Qiumin Wu , Benxian Shen , Hui Sun
|
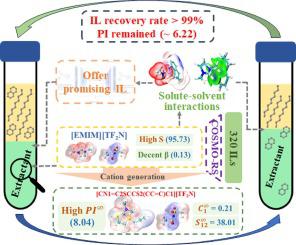
The separation of aromatic hydrocarbons from petroleum fractions is of great significance for both the quality upgrading of products and value-added utilization of aromatic compounds. However, the separation of polycyclic aromatic hydrocarbons (PAHs) and their derivatives from diesel remains challenging because of their lower solubility and selectivity as compared to the monocyclic aromatic compounds. In this work, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM][TFN]) was screened from 320 ionic liquid (IL) candidates through solvent capacity predication using COSMO-RS method. Liquid-liquid equilibrium experiments were carried out using a series of tetralin/n-decane mixtures with different tetralin concentrations to evaluate the extraction performance of several solvents. The results indicate that [EMIM][TFN] has high selectivity up to 95.73 for tetralin over n-decane, which is 4.5 and 3.9 times higher than that of dimethyl sulfoxide and sulfolane. During five regeneration cycles, the [EMIM][TFN] exhibits consistent extraction performance and nearly complete recovery. Molecular polarity index (MPI) analysis indicates that tetralin has larger dissolving affinity to solvents as compared to n-decane. Combined analyses of interaction energy, energy decomposition, and visualization of weak interaction reveal that the crucial role of weak hydrogen bond in dominating the complex solvent–solute interactions. Compared to n-decane, tetralin shows a stronger interaction with the solvents due to the existence of C-H···π and hydrogen bond interactions. The great difference in interaction energy between {[EMIM] + tetralin} and {[EMIM] + n-decane}, therefore, endows [EMIM][TFN] with high selectivity for tetralin. Given the dominant role of cation, a IL, [CN1 = C2SCCS2(CC = C)C1][TFN] having both high selectivity and high solvent capacity was subsequently explored using the cation generation method based on the SeqVAE model. Furthermore, present study proposes a strategy for the design of ILs for separation and other specific application circumstances.
中文翻译:

通过多重相互作用分析和实验评估探索离子液体从柴油中萃取分离芳香烃
从石油馏分中分离芳烃对于产品质量升级和芳烃化合物的增值利用都具有重要意义。然而,从柴油中分离多环芳烃(PAH)及其衍生物仍然具有挑战性,因为与单环芳族化合物相比,它们的溶解度和选择性较低。在这项工作中,使用 COSMO-RS 方法通过溶剂容量预测从 320 种离子液体 (IL) 候选物中筛选出 1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺 ([EMIM][TFN])。使用一系列不同四氢化萘浓度的四氢化萘/正癸烷混合物进行液-液平衡实验,以评估几种溶剂的萃取性能。结果表明,[EMIM][TFN]对四氢萘对正癸烷的选择性高达95.73,分别是二甲亚砜和环丁砜的4.5和3.9倍。在五个再生周期中,[EMIM][TFN] 表现出一致的萃取性能和几乎完全恢复。分子极性指数(MPI)分析表明,与正癸烷相比,四氢化萘对溶剂具有更大的溶解亲和力。相互作用能、能量分解和弱相互作用可视化的综合分析揭示了弱氢键在主导复杂溶剂-溶质相互作用中的关键作用。与正癸烷相比,由于CH·π和氢键相互作用的存在,四氢化萘与溶剂表现出更强的相互作用。因此,{[EMIM] + 四氢化萘}和{[EMIM] + 正癸烷}之间相互作用能的巨大差异赋予[EMIM][TFN]对四氢化萘的高选择性。鉴于阳离子的主导作用,随后使用基于 SeqVAE 模型的阳离子生成方法探索了具有高选择性和高溶剂容量的 IL,[CN1 = C2SCCS2(CC = C)C1][TFN]。此外,本研究提出了用于分离和其他特定应用环境的离子液体的设计策略。
更新日期:2024-04-05
中文翻译:

通过多重相互作用分析和实验评估探索离子液体从柴油中萃取分离芳香烃
从石油馏分中分离芳烃对于产品质量升级和芳烃化合物的增值利用都具有重要意义。然而,从柴油中分离多环芳烃(PAH)及其衍生物仍然具有挑战性,因为与单环芳族化合物相比,它们的溶解度和选择性较低。在这项工作中,使用 COSMO-RS 方法通过溶剂容量预测从 320 种离子液体 (IL) 候选物中筛选出 1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺 ([EMIM][TFN])。使用一系列不同四氢化萘浓度的四氢化萘/正癸烷混合物进行液-液平衡实验,以评估几种溶剂的萃取性能。结果表明,[EMIM][TFN]对四氢萘对正癸烷的选择性高达95.73,分别是二甲亚砜和环丁砜的4.5和3.9倍。在五个再生周期中,[EMIM][TFN] 表现出一致的萃取性能和几乎完全恢复。分子极性指数(MPI)分析表明,与正癸烷相比,四氢化萘对溶剂具有更大的溶解亲和力。相互作用能、能量分解和弱相互作用可视化的综合分析揭示了弱氢键在主导复杂溶剂-溶质相互作用中的关键作用。与正癸烷相比,由于CH·π和氢键相互作用的存在,四氢化萘与溶剂表现出更强的相互作用。因此,{[EMIM] + 四氢化萘}和{[EMIM] + 正癸烷}之间相互作用能的巨大差异赋予[EMIM][TFN]对四氢化萘的高选择性。鉴于阳离子的主导作用,随后使用基于 SeqVAE 模型的阳离子生成方法探索了具有高选择性和高溶剂容量的 IL,[CN1 = C2SCCS2(CC = C)C1][TFN]。此外,本研究提出了用于分离和其他特定应用环境的离子液体的设计策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号