当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
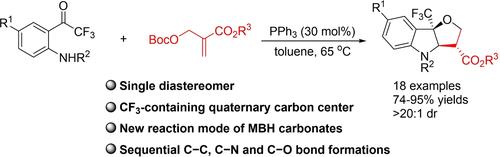
Phosphine-Catalyzed Domino Annulation Reactions of o-Aminotrifluoroacetophenone Derivatives with Morita–Baylis–Hillman Carbonates: Construction of Tetrahydrofuro[3,2-b]indolines
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-04-04 , DOI: 10.1002/adsc.202400205 Yannan Zhu 1 , He Zhao 1 , Qiuyun Li 1 , Bo Fang 2 , Yining Wang 1 , Gang Qi 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-04-04 , DOI: 10.1002/adsc.202400205 Yannan Zhu 1 , He Zhao 1 , Qiuyun Li 1 , Bo Fang 2 , Yining Wang 1 , Gang Qi 1
Affiliation
|
The domino annulation reactions of <i>o</i>‐aminotrifluoroacetophenone derivatives and MBH carbonates enabled by PPh<sub>3</sub> have been reported, which provide a series of tetrahydrofuro[3,2‐b]indolines containing a CF<sub>3</sub>‐substituted tetrasubstituted carbon stereocenters in yields of 74 to 95% and >20:1 dr. In this process, MBH carbonates were used as C1/C3 synthons. The MTT assay shows that the products exhibit the activity of inhibitory effect on proliferation on Hela cancer cell line.
中文翻译:

磷化氢催化邻氨基三氟苯乙酮衍生物与 Morita-Baylis-Hillman 碳酸盐的多米诺环化反应:四氢呋喃[3,2-b]二氢吲哚的构建
已经报道了由 PPh<sub>3</sub> 实现的 <i>o</i>-氨基三氟苯乙酮衍生物和 MBH 碳酸盐的多米诺环化反应,提供了一系列含有CF<sub>3</sub>-取代的四取代碳立构中心,产率为 74% 至 95%,且 Dr. >20:1。在此过程中,MBH 碳酸盐用作 C1/C3 合成子。 MTT法检测显示产物对Hela癌细胞系具有增殖抑制活性。
更新日期:2024-04-04
中文翻译:

磷化氢催化邻氨基三氟苯乙酮衍生物与 Morita-Baylis-Hillman 碳酸盐的多米诺环化反应:四氢呋喃[3,2-b]二氢吲哚的构建
已经报道了由 PPh<sub>3</sub> 实现的 <i>o</i>-氨基三氟苯乙酮衍生物和 MBH 碳酸盐的多米诺环化反应,提供了一系列含有CF<sub>3</sub>-取代的四取代碳立构中心,产率为 74% 至 95%,且 Dr. >20:1。在此过程中,MBH 碳酸盐用作 C1/C3 合成子。 MTT法检测显示产物对Hela癌细胞系具有增殖抑制活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号