当前位置:
X-MOL 学术
›
Immun. Inflamm. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nirmatrelvir and ritonavir combination against COVID‐19 caused by omicron BA.2.2 in the elderly: A single‐center large observational study
Immunity, Inflammation and Disease ( IF 2.493 ) Pub Date : 2024-04-05 , DOI: 10.1002/iid3.1232 Can Chen 1 , Ranyi Li 1 , Shuliang Xing 2 , Lei Cao 3 , Yue Qu 4 , Qianzhou Lv 1 , Xiaoyu Li 1 , Zhangzhang Chen 1
Immunity, Inflammation and Disease ( IF 2.493 ) Pub Date : 2024-04-05 , DOI: 10.1002/iid3.1232 Can Chen 1 , Ranyi Li 1 , Shuliang Xing 2 , Lei Cao 3 , Yue Qu 4 , Qianzhou Lv 1 , Xiaoyu Li 1 , Zhangzhang Chen 1
Affiliation
|
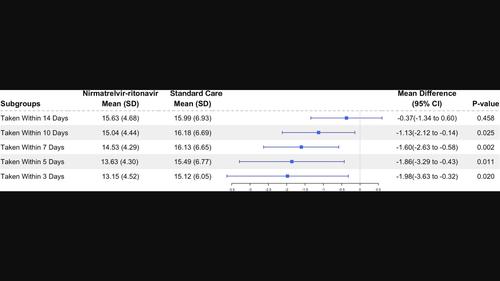
BackgroundSince coronavirus 2019 (COVID‐19) swept the world, a variety of novel therapeutic and prevention strategies have been developed, among which nirmatrelvir–ritonavir is highly recommended. We intended to assess the effectiveness and safety of nirmatrelvir–ritonavir in the elderly mild‐to‐moderate COVID‐19 population caused by the omicron BA.2.2 variant in real‐world settings.MethodsAn observational study was conducted retrospectively to review the outcomes of mild‐to‐moderate COVID‐19 patients admitted between April 26 and June 30, 2022. Patients' baseline characteristics were collected and assessed. Participants in the intervention group were administered nirmatrelvir–ritonavir in addition to standard care, whereas those in the control group only received standard care. The primary outcome was the duration between the initial positive reverse‐transcription polymerase chain reaction (RT‐PCR) test and the subsequent conversion to a negative result.ResultsThe analysis included 324 patients who were administered nirmatrelvir–ritonavir and an equal number of control patients. The patient characteristics in both groups were evenly matched. The average duration from the initial positive RT‐PCR to negative conversion was similar in both groups (16.2 ± 5.0 vs. 16.1 ± 6.3 days, p = .83). Control patients exhibited slower conversion in comparison to patients who received nirmatrelvir–ritonavir treatment within 10 days of symptom onset.ConclusionsThese findings suggest that administering nirmatrelvir–ritonavir within 10 days of symptom onset could potentially reduce the time it takes for SARS‐CoV‐2‐infected patients to negative RT‐PCR results, thereby expanding the current usage guidelines for nirmatrelvir–ritonavir.
中文翻译:

尼马曲韦和利托那韦联合治疗老年人 omicron BA.2.2 引起的 COVID-19:一项单中心大型观察性研究
背景自2019冠状病毒(COVID-19)席卷全球以来,已经开发出多种新型治疗和预防策略,其中强烈推荐尼马曲韦-利托那韦。我们打算在现实环境中评估尼马瑞韦-利托那韦在由 omicron BA.2.2 变体引起的老年轻至中度 COVID-19 人群中的有效性和安全性。 2022 年 4 月 26 日至 6 月 30 日期间入院的 ‐ 至中度 COVID-19 患者。收集并评估患者的基线特征。干预组的参与者除了标准护理外还接受尼马瑞韦-利托那韦治疗,而对照组的参与者仅接受标准护理。主要结局是最初的逆转录聚合酶链反应 (RT-PCR) 检测呈阳性与随后转为阴性结果之间的持续时间。 结果分析包括 324 名接受尼马瑞韦-利托那韦治疗的患者和同等数量的对照患者。两组患者特征均匀匹配。两组从最初的 RT-PCR 阳性到转阴的平均持续时间相似(16.2 ± 5.0 天与 16.1 ± 6.3 天,p = .83)。与症状出现 10 天内接受尼马瑞韦-利托那韦治疗的患者相比,对照患者的转化速度较慢。结论这些研究结果表明,在症状出现 10 天内服用尼马瑞韦-利托那韦可能会缩短 SARS-CoV-2- 的治疗时间。感染患者的 RT-PCR 结果呈阴性,从而扩大了目前尼马瑞韦-利托那韦的使用指南。
更新日期:2024-04-05
中文翻译:

尼马曲韦和利托那韦联合治疗老年人 omicron BA.2.2 引起的 COVID-19:一项单中心大型观察性研究
背景自2019冠状病毒(COVID-19)席卷全球以来,已经开发出多种新型治疗和预防策略,其中强烈推荐尼马曲韦-利托那韦。我们打算在现实环境中评估尼马瑞韦-利托那韦在由 omicron BA.2.2 变体引起的老年轻至中度 COVID-19 人群中的有效性和安全性。 2022 年 4 月 26 日至 6 月 30 日期间入院的 ‐ 至中度 COVID-19 患者。收集并评估患者的基线特征。干预组的参与者除了标准护理外还接受尼马瑞韦-利托那韦治疗,而对照组的参与者仅接受标准护理。主要结局是最初的逆转录聚合酶链反应 (RT-PCR) 检测呈阳性与随后转为阴性结果之间的持续时间。 结果分析包括 324 名接受尼马瑞韦-利托那韦治疗的患者和同等数量的对照患者。两组患者特征均匀匹配。两组从最初的 RT-PCR 阳性到转阴的平均持续时间相似(16.2 ± 5.0 天与 16.1 ± 6.3 天,



























 京公网安备 11010802027423号
京公网安备 11010802027423号