当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Detailed DFT/MD simulation, QSAR modeling, electrochemical, and surface morphological studies of self-assembled surfactants as eco-friendly corrosion inhibitors for copper in 1 M HNO3 solution
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2024-04-02 , DOI: 10.1016/j.jiec.2024.03.056 Ahmed A. Farag , Salah M. Tawfik , Ali A. Abd-Elaal , N.S. Abdelshafi
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2024-04-02 , DOI: 10.1016/j.jiec.2024.03.056 Ahmed A. Farag , Salah M. Tawfik , Ali A. Abd-Elaal , N.S. Abdelshafi
|
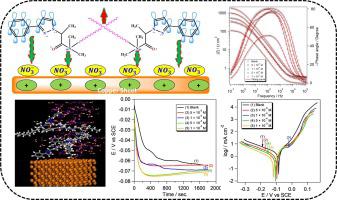
Three novel self-assembled phenyl pyrazole cationic surfactant derivatives, coded as SA-6, SA-12, and SA-18, were synthesized, structure confirmed, and examined as eco-friendly corrosion inhibitors for Cu in 1 M HNO electrolyte using open circuit potential (OCP), potentiodynamic polarization (PDP), and electrochemical impedance spectroscopy (EIS) measurements. The efficacy of these surfactants was pointedly influenced by dosage (5 × 10 M − 1 × 10 M), attaining a maximum of 90.9 %, 92.9 %, and 94.5 % for SA-6, SA-12, and SA-18, respectively, at 1 × 10 M. The fact that the highest shift in corrosion potential () is so minor suggests that the compounds under investigation are inhibitors of the mixed type (anodic/cathodic). The high value of and the negative value (−35.0, −35.8, and −36.8 kJ mol) suggests that the SA-6, SA-12, and SA-18 inhibitors have a strong and spontaneous tendency to adsorbed onto the Cu substrate. The development of the self-assembled surfactant defensive film on the Cu substrate was morphologically verified by atomic force microscopy (AFM). The studied self-assembled surfactants adsorbed physically and chemically according to Langmuir isotherm model. Quantitative structure–activity relationship (QSAR) was used to construct the prediction model for SA-6, SA-12, and SA-18′s anti-corrosion properties, while density functional theory (DFT) calculations were used to determine the chemical descriptors linked to the frontier molecular orbitals (FMOs). It was shown that the results of the computational and electrochemical experiments were highly consistent.
中文翻译:

自组装表面活性剂作为铜在 1 M HNO3 溶液中的环保腐蚀抑制剂的详细 DFT/MD 模拟、QSAR 建模、电化学和表面形态研究
合成了三种新型自组装苯基吡唑阳离子表面活性剂衍生物(编号为 SA-6、SA-12 和 SA-18),并对其结构进行了确认,并使用开路测试作为 1 M HNO 电解液中 Cu 的环保缓蚀剂电势 (OCP)、动电位极化 (PDP) 和电化学阻抗谱 (EIS) 测量。这些表面活性剂的功效明显受到剂量 (5 × 10 M − 1 × 10 M) 的影响,SA-6、SA-12 和 SA-18 的最大值分别为 90.9 %、92.9 % 和 94.5 % ,在 1 × 10 M 处。腐蚀电位 () 的最高变化非常小,这一事实表明所研究的化合物是混合类型(阳极/阴极)的抑制剂。的高值和负值(-35.0、-35.8 和 -36.8 kJ mol)表明 SA-6、SA-12 和 SA-18 抑制剂具有强烈且自发的吸附到 Cu 底物上的倾向。通过原子力显微镜(AFM)对铜基板上自组装表面活性剂防御膜的形成进行了形态学验证。所研究的自组装表面活性剂根据Langmuir等温线模型进行物理和化学吸附。使用定量构效关系(QSAR)构建SA-6、SA-12和SA-18的防腐性能预测模型,同时使用密度泛函理论(DFT)计算来确定化学描述符与前沿分子轨道(FMO)相连。结果表明,计算结果与电化学实验结果高度一致。
更新日期:2024-04-02
中文翻译:

自组装表面活性剂作为铜在 1 M HNO3 溶液中的环保腐蚀抑制剂的详细 DFT/MD 模拟、QSAR 建模、电化学和表面形态研究
合成了三种新型自组装苯基吡唑阳离子表面活性剂衍生物(编号为 SA-6、SA-12 和 SA-18),并对其结构进行了确认,并使用开路测试作为 1 M HNO 电解液中 Cu 的环保缓蚀剂电势 (OCP)、动电位极化 (PDP) 和电化学阻抗谱 (EIS) 测量。这些表面活性剂的功效明显受到剂量 (5 × 10 M − 1 × 10 M) 的影响,SA-6、SA-12 和 SA-18 的最大值分别为 90.9 %、92.9 % 和 94.5 % ,在 1 × 10 M 处。腐蚀电位 () 的最高变化非常小,这一事实表明所研究的化合物是混合类型(阳极/阴极)的抑制剂。的高值和负值(-35.0、-35.8 和 -36.8 kJ mol)表明 SA-6、SA-12 和 SA-18 抑制剂具有强烈且自发的吸附到 Cu 底物上的倾向。通过原子力显微镜(AFM)对铜基板上自组装表面活性剂防御膜的形成进行了形态学验证。所研究的自组装表面活性剂根据Langmuir等温线模型进行物理和化学吸附。使用定量构效关系(QSAR)构建SA-6、SA-12和SA-18的防腐性能预测模型,同时使用密度泛函理论(DFT)计算来确定化学描述符与前沿分子轨道(FMO)相连。结果表明,计算结果与电化学实验结果高度一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号