当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibitor binding to metal-substituted metalloenzyme: Sulfonamide affinity for carbonic anhydrase IX
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2024-04-01 , DOI: 10.1016/j.jinorgbio.2024.112547 Denis Baronas , Birutė Knašienė , Aurelija Mickevičiūtė , Jelena Jachno , Evaldas Naujalis , Asta Zubrienė , Daumantas Matulis
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2024-04-01 , DOI: 10.1016/j.jinorgbio.2024.112547 Denis Baronas , Birutė Knašienė , Aurelija Mickevičiūtė , Jelena Jachno , Evaldas Naujalis , Asta Zubrienė , Daumantas Matulis
|
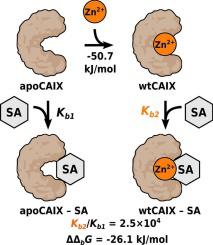
Transition metal ions are structural and catalytic cofactors of many proteins including human carbonic anhydrase (CA), a Zn-dependent hydrolase. Sulfonamide inhibitors of CA recognize and form a coordination bond with the Zn ion located in the active site of the enzyme. The Zn ion may be removed or substituted with other metal ions. Such CA protein retains the structure and could serve as a tool to study metal ion role in the recognition and binding affinity of inhibitor molecules. We measured the affinities of selected divalent transition metal ions, including Mn, Fe, Co, Ni, Cu, Cd, Hg, and Zn to metal-free CA isozymes CA I, CA II, and CAIX by fluorescence-based thermal shift assay, prepared metal-substituted CAs, and determined binding of diverse sulfonamide compounds.
中文翻译:

与金属取代的金属酶结合的抑制剂:磺酰胺对碳酸酐酶 IX 的亲和力
过渡金属离子是许多蛋白质的结构和催化辅助因子,包括人碳酸酐酶 (CA)(一种锌依赖性水解酶)。 CA 的磺酰胺抑制剂可识别酶活性位点的 Zn 离子并与其形成配位键。 Zn离子可被除去或用其他金属离子取代。这种CA蛋白保留了结构,可以作为研究金属离子在抑制剂分子的识别和结合亲和力中的作用的工具。我们通过基于荧光的热位移测定测量了选定的二价过渡金属离子(包括 Mn、Fe、Co、Ni、Cu、Cd、Hg 和 Zn)与无金属 CA 同工酶 CA I、CA II 和 CAIX 的亲和力,制备了金属取代的 CA,并测定了多种磺酰胺化合物的结合。
更新日期:2024-04-01
中文翻译:

与金属取代的金属酶结合的抑制剂:磺酰胺对碳酸酐酶 IX 的亲和力
过渡金属离子是许多蛋白质的结构和催化辅助因子,包括人碳酸酐酶 (CA)(一种锌依赖性水解酶)。 CA 的磺酰胺抑制剂可识别酶活性位点的 Zn 离子并与其形成配位键。 Zn离子可被除去或用其他金属离子取代。这种CA蛋白保留了结构,可以作为研究金属离子在抑制剂分子的识别和结合亲和力中的作用的工具。我们通过基于荧光的热位移测定测量了选定的二价过渡金属离子(包括 Mn、Fe、Co、Ni、Cu、Cd、Hg 和 Zn)与无金属 CA 同工酶 CA I、CA II 和 CAIX 的亲和力,制备了金属取代的 CA,并测定了多种磺酰胺化合物的结合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号