当前位置:
X-MOL 学术
›
Int. J. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Integration of caveolin-mediated cytosolic delivery and enzyme-responsive releasing of squalenoyl nanoparticles enhance the anti-cancer efficacy of chidamide in pancreatic cancer
International Journal of Pharmaceutics ( IF 5.8 ) Pub Date : 2024-03-30 , DOI: 10.1016/j.ijpharm.2024.124072 Junyan Chen , Kaidi Chen , Shuai Xue , Xiao Cheng , Yuwei Qi , Hangjie Wang , Wei Li , Guilin Cheng , Yang Xiong , Chaofeng Mu , Mancang Gu
International Journal of Pharmaceutics ( IF 5.8 ) Pub Date : 2024-03-30 , DOI: 10.1016/j.ijpharm.2024.124072 Junyan Chen , Kaidi Chen , Shuai Xue , Xiao Cheng , Yuwei Qi , Hangjie Wang , Wei Li , Guilin Cheng , Yang Xiong , Chaofeng Mu , Mancang Gu
|
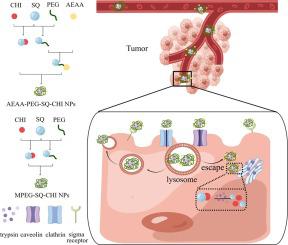
We explored the potential of overcoming the dense interstitial barrier in pancreatic cancer treatment by enhancing the uptake of hydrophilic chemotherapeutic drugs. In this study, we synthesized the squalenoyl-chidamide prodrug (SQ-CHI), linking lipophilic squalene (SQ) with the hydrophilic antitumor drug chidamide (CHI) through a trypsin-responsive bond. Self-assembled nanoparticles with sigma receptor-bound aminoethyl anisamide (AEAA) modification, forming AEAA-PEG-SQ-CHI NPs (A-C NPs, size 116.6 ± 0.4 nm), and reference nanoparticles without AEAA modification, forming mPEG-SQ-CHI NPs (M−C NPs, size 88.3 ± 0.3 nm), were prepared. A-C NPs exhibited significantly higher CHI release (74.7 %) in 0.5 % trypsin medium compared to release (20.2 %) in medium without trypsin. cell uptake assays revealed 3.6 and 2.3times higher permeation of A-C NPs into tumorspheres of PSN-1/HPSC or CFPAC-1/HPSC, respectively, compared to M−C NPs. Following intraperitoneal administration to subcutaneous tumor-bearing nude mice, the A-C NPs group demonstrated significant anti-pancreatic cancer efficacy, inducing cancer cell apoptosis and inhibiting proliferation . Mechanistic studies revealed that AEAA surface modification on nanoparticles promoted intracellular uptake through caveolin-mediated endocytosis. This nanoparticle system presents a novel therapeutic approach for pancreatic cancer treatment, offering a delivery strategy to enhance efficacy through improved tumor permeation, trypsin-responsive drug release, and specific cell surface receptor-mediated intracellular uptake.
中文翻译:

小窝蛋白介导的胞质递送和角鲨烯酰纳米颗粒的酶响应释放的整合增强了西达本胺在胰腺癌中的抗癌功效
我们探索了通过增强亲水性化疗药物的摄取来克服胰腺癌治疗中致密间质屏障的潜力。在这项研究中,我们合成了角鲨烯酰西达本胺前药(SQ-CHI),通过胰蛋白酶响应键将亲脂性角鲨烯(SQ)与亲水性抗肿瘤药物西达本胺(CHI)连接起来。具有 σ 受体结合氨基乙基茴香酰胺 (AEAA) 修饰的自组装纳米颗粒,形成 AEAA-PEG-SQ-CHI NP(AC NP,尺寸 116.6 ± 0.4 nm),以及未经 AEAA 修饰的参考纳米颗粒,形成 mPEG-SQ-CHI NP制备了(M−C NP,尺寸 88.3 ± 0.3 nm)。与不含胰蛋白酶的培养基中的释放 (20.2%) 相比,AC NP 在 0.5% 胰蛋白酶培养基中表现出显着更高的 CHI 释放 (74.7%)。细胞摄取测定显示,与 M−C NP 相比,AC NP 对 PSN-1/HPSC 或 CFPAC-1/HPSC 肿瘤球的渗透率分别高 3.6 倍和 2.3 倍。对皮下荷瘤裸鼠腹腔给药后,AC NPs组表现出显着的抗胰腺癌功效,诱导癌细胞凋亡并抑制增殖。机理研究表明,AEAA 纳米颗粒表面修饰通过小窝蛋白介导的内吞作用促进细胞内摄取。该纳米颗粒系统为胰腺癌治疗提供了一种新颖的治疗方法,提供了一种通过改善肿瘤渗透、胰蛋白酶响应性药物释放和特定细胞表面受体介导的细胞内摄取来增强疗效的递送策略。
更新日期:2024-03-30
中文翻译:

小窝蛋白介导的胞质递送和角鲨烯酰纳米颗粒的酶响应释放的整合增强了西达本胺在胰腺癌中的抗癌功效
我们探索了通过增强亲水性化疗药物的摄取来克服胰腺癌治疗中致密间质屏障的潜力。在这项研究中,我们合成了角鲨烯酰西达本胺前药(SQ-CHI),通过胰蛋白酶响应键将亲脂性角鲨烯(SQ)与亲水性抗肿瘤药物西达本胺(CHI)连接起来。具有 σ 受体结合氨基乙基茴香酰胺 (AEAA) 修饰的自组装纳米颗粒,形成 AEAA-PEG-SQ-CHI NP(AC NP,尺寸 116.6 ± 0.4 nm),以及未经 AEAA 修饰的参考纳米颗粒,形成 mPEG-SQ-CHI NP制备了(M−C NP,尺寸 88.3 ± 0.3 nm)。与不含胰蛋白酶的培养基中的释放 (20.2%) 相比,AC NP 在 0.5% 胰蛋白酶培养基中表现出显着更高的 CHI 释放 (74.7%)。细胞摄取测定显示,与 M−C NP 相比,AC NP 对 PSN-1/HPSC 或 CFPAC-1/HPSC 肿瘤球的渗透率分别高 3.6 倍和 2.3 倍。对皮下荷瘤裸鼠腹腔给药后,AC NPs组表现出显着的抗胰腺癌功效,诱导癌细胞凋亡并抑制增殖。机理研究表明,AEAA 纳米颗粒表面修饰通过小窝蛋白介导的内吞作用促进细胞内摄取。该纳米颗粒系统为胰腺癌治疗提供了一种新颖的治疗方法,提供了一种通过改善肿瘤渗透、胰蛋白酶响应性药物释放和特定细胞表面受体介导的细胞内摄取来增强疗效的递送策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号