Acta Neurologica Belgica ( IF 2.7 ) Pub Date : 2024-04-06 , DOI: 10.1007/s13760-024-02508-x Shirin Mavandadi , Sepideh Paybast , Monirsadat Mirzadeh , Hossein Mozhdehipanah
|
Introduction
Fatigue is a highly prevalent debilitating symptom among patients with multiple sclerosis (PwMS), which markedly affects the quality of life. The present study aimed to evaluate the effect of extended-release fampridine on fatigue in PwMS.
Methods
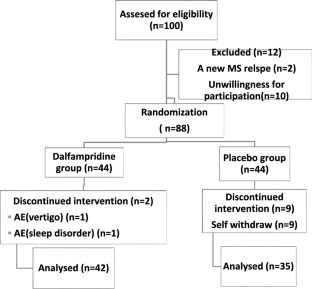
This was a randomized, double-blind clinical trial on 77 PwMS with a complaint of fatigue, aged over 18 years old, randomized to extended-release fampridine (n = 44) or placebo (n = 35) for 12 weeks. Fatigue and motor function were assessed at baseline and end point.
Results
A total of 88 patients were recruited, of whom 77 were analyzed. 80.5% were female, with a median age of 38. 87% were diagnosed with relapsing–remitting MS (RRMS) with a median disease duration of 96 months. Fingolimod (37.7%) was considered the most frequently used DMT, followed by ani-CD20s (32.5%). The total median MFIS score was 43.5 and 37 in the fampridine and placebo groups which were not significantly different (p > 0.05). After 12 weeks, the total MFIS improved in both groups compared to the baseline, which was significant in the active group (p = 0.04). However, the final end point total MFIS was still comparable between the two groups (p = 0.11).
Conclusion
The present study revealed a positive short-term effect of extended-release fampridine on MFIS in PwMS. However, this effect was not significantly superior to the placebo.
中文翻译:

达芬吡啶治疗多发性硬化症患者原发性疲劳:一项随机临床试验
介绍
疲劳是多发性硬化症 (PwMS) 患者中一种非常普遍的衰弱症状,显着影响生活质量。本研究旨在评估缓释芬吡啶对 PwMS 疲劳的影响。
方法
这是一项针对 77 名年龄超过 18 岁、主诉疲劳的 PwMS 的随机、双盲临床试验,随机分配至缓释 fampridine ( n = 44) 或安慰剂 ( n = 35),为期 12 周。在基线和终点评估疲劳和运动功能。
结果
总共招募了 88 名患者,其中 77 名进行了分析。 80.5% 为女性,中位年龄为 38 岁。87% 被诊断患有复发缓解型 MS (RRMS),中位病程为 96 个月。芬戈莫德 (37.7%) 被认为是最常用的 DMT,其次是 ani-CD20 (32.5%)。芬吡啶组和安慰剂组的 MFIS 总中位分分别为 43.5 分和 37 分,没有显着差异 ( p > 0.05)。 12 周后,与基线相比,两组的总 MFIS 均有所改善,这在活动组中显着 ( p = 0.04)。然而,最终终点总 MFIS 在两组之间仍然具有可比性 ( p = 0.11)。
结论
本研究揭示了缓释 fampridine 对 PwMS 中 MFIS 的积极短期影响。然而,这种效果并不明显优于安慰剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号