当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Investigations of Phthalazinone Derivatives as Allosteric Inhibitors of Human DNA Methyltransferase 3A
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2024-04-08 , DOI: 10.1021/acsmedchemlett.3c00528 Ivan Hernandez 1 , Ethan Ward 1 , Thomas R. R. Pettus 1 , Norbert O. Reich 1, 2
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2024-04-08 , DOI: 10.1021/acsmedchemlett.3c00528 Ivan Hernandez 1 , Ethan Ward 1 , Thomas R. R. Pettus 1 , Norbert O. Reich 1, 2
Affiliation
|
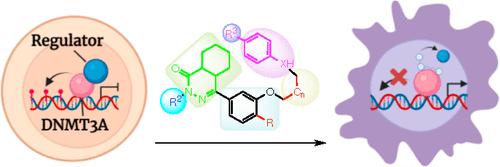
The development of new therapeutics targeting enzymes involved in epigenetic pathways such as histone modification and DNA methylation has received a lot of attention, particularly for targeting diverse cancers. Unfortunately, irreversible nucleoside inhibitors (azacytidine and decitabine) have proven highly cytotoxic, and competitive inhibitors are also problematic. This work describes synthetic and structural investigations of a new class of allosteric DNA methyltransferase 3A (DNMT3A) inhibitors, leading to the identification of several critical pharmacophores in the lead structure. Specifically, we find that the tetrazole and phthalazinone moieties are indispensable for the inhibitory activity of DNMT3A and elucidate other modifiable regions in the lead compound.
中文翻译:

作为人类 DNA 甲基转移酶 3A 变构抑制剂的酞嗪酮衍生物的结构研究
针对组蛋白修饰和 DNA 甲基化等表观遗传途径中涉及的酶的新疗法的开发受到了广泛关注,特别是针对多种癌症。不幸的是,不可逆核苷抑制剂(氮胞苷和地西他滨)已被证明具有高度细胞毒性,并且竞争性抑制剂也存在问题。这项工作描述了新型变构 DNA 甲基转移酶 3A (DNMT3A) 抑制剂的合成和结构研究,从而鉴定了先导结构中的几个关键药效团。具体来说,我们发现四唑和二氮杂萘酮部分对于 DNMT3A 的抑制活性是不可或缺的,并阐明了先导化合物中的其他可修饰区域。
更新日期:2024-04-08
中文翻译:

作为人类 DNA 甲基转移酶 3A 变构抑制剂的酞嗪酮衍生物的结构研究
针对组蛋白修饰和 DNA 甲基化等表观遗传途径中涉及的酶的新疗法的开发受到了广泛关注,特别是针对多种癌症。不幸的是,不可逆核苷抑制剂(氮胞苷和地西他滨)已被证明具有高度细胞毒性,并且竞争性抑制剂也存在问题。这项工作描述了新型变构 DNA 甲基转移酶 3A (DNMT3A) 抑制剂的合成和结构研究,从而鉴定了先导结构中的几个关键药效团。具体来说,我们发现四唑和二氮杂萘酮部分对于 DNMT3A 的抑制活性是不可或缺的,并阐明了先导化合物中的其他可修饰区域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号