Chemical & Pharmaceutical Bulletin ( IF 1.7 ) Pub Date : 2024-04-03 , DOI: 10.1248/cpb.c24-00126 Hiroyuki Mutoh 1 , Yuuki Watanabe 1 , Daiki Kamakura 1 , Koichi Hagiwara 1 , Masayuki Inoue 1
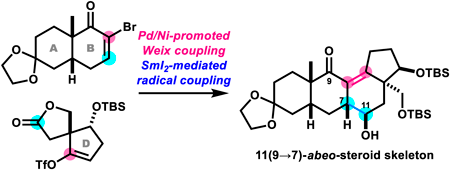
Batrachotoxin (1) is a potent cardio- and neurotoxic steroid isolated from certain species of frogs, birds, and beetles. We previously disclosed two synthetic routes to 1. During our synthetic studies toward 1, we explored an alternative strategy for efficiently assembling its 6/6/6/5-membered steroidal skeleton (ABCD-ring). Here we report the application of intermolecular Weix and intramolecular pinacol coupling reactions. While Pd/Ni-promoted Weix coupling linked the AB-ring and D-ring fragments, SmI2-mediated pinacol coupling did not cyclize the C-ring. Instead, we discovered that SmI2 promoted a 1,4-addition of the α-alkoxy radical intermediate to produce the unusual 11(9→7)-abeo-steroid skeleton. Thus, this study demonstrates the convergent assembly of the skeleton of the natural product matsutakone in 11 steps from 2-allyl-3-hydroxycyclopent-2-en-1-one.
 Fullsize Image
Fullsize Image
中文翻译:

蝙蝠毒素合成研究中 11(9→7)-abeo-类固醇骨架的意外形成
蝙蝠毒素 (1) 是一种强效心脏和神经毒性类固醇,从某些种类的青蛙、鸟类和甲虫中分离出来。我们之前公开了1的两条合成路线。在我们对1的合成研究中,我们探索了一种有效组装其6/6/6/5元甾体骨架(ABCD环)的替代策略。在这里,我们报道了分子间Weix和分子内频哪醇偶联反应的应用。虽然Pd/Ni促进的Weix偶联连接AB环和D环片段,但SmI 2介导的频哪醇偶联并不环化C环。相反,我们发现 SmI 2促进 α-烷氧基自由基中间体的 1,4-加成,产生不寻常的 11(9→7)- abeo -类固醇骨架。因此,本研究证明了天然产物 matsutakone 骨架从 2-烯丙基-3-羟基环戊-2-en-1-one 的 11 个步骤的聚合组装。
 全尺寸图像
全尺寸图像



























 京公网安备 11010802027423号
京公网安备 11010802027423号