当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into the mechanism of simultaneous removal of NOx and toluene by MnCuTi ternary catalyst
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.mcat.2024.114110 Haoxu Ji , Yaqin Hou , Biao Li , Yatao Yang , Shuang Ma , Zhanggen Huang
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.mcat.2024.114110 Haoxu Ji , Yaqin Hou , Biao Li , Yatao Yang , Shuang Ma , Zhanggen Huang
|
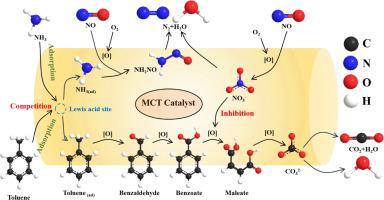
At present, the emission of flue gas from the high-energy-consuming non-electric industries, represented by iron and steel, coking and cement, has become the main source of air pollution in China. Among these, volatile organic compounds (VOCs) and nitrogen oxides (NO) are the main precursor for PM and ozone pollution. Herein, a series of MnCuTi ternary system catalysts with different Mn-Cu molar ratios were synthesized using the sol-gel method for the simultaneous removal of NO and toluene. Combined with the catalytic performance and physicochemical properties, the results showed that the MnCuTi ternary catalysts possessed a large specific surface area and abundant mesopore structure. The synergistic effect between manganese and copper promoted the cyclic electron migration over the redox reactions, and improved the synergistic reaction performance of NO reduction and toluene oxidation. Among different catalysts, 1.5MCT catalyst showed the best synergic activity, with 87 % NO conversion and 92 % toluene conversion at 240 °C. The interaction mechanism and the evolution of intermediate species in the collaborative reaction process were thoroughly revealed by in situ DRIFTS, it is discovered that NH and NO inhibit the toluene oxidation due to the competitive adsorption and the nitrate deposition. However, when NH and NOx were introduced concurrently, they preferentially generated NHNO, thereby diminishing their inhibitory effect. This study provided theoretical basis and technical support for multi-pollutant collaborative control in steel/coking industry.
中文翻译:

深入探讨MnCuTi三元催化剂同时脱除NOx和甲苯的机理
目前,以钢铁、焦化、水泥为代表的高耗能非电行业烟气排放已成为我国大气污染的主要来源。其中,挥发性有机化合物(VOC)和氮氧化物(NO)是PM和臭氧污染的主要前体。采用溶胶-凝胶法合成了一系列不同Mn-Cu摩尔比的MnCuTi三元体系催化剂,用于同时脱除NO和甲苯。结合催化性能和理化性质,结果表明MnCuTi三元催化剂具有较大的比表面积和丰富的介孔结构。锰和铜之间的协同作用促进了氧化还原反应的循环电子迁移,提高了NO还原和甲苯氧化的协同反应性能。在不同的催化剂中,1.5MCT催化剂表现出最好的协同活性,在240℃时NO转化率为87%,甲苯转化率为92%。原位DRIFTS彻底揭示了协同反应过程中的相互作用机制和中间物种的演化,发现NH和NO通过竞争吸附和硝酸盐沉积抑制了甲苯氧化。然而,当同时引入NH和NOx时,它们优先生成NHNO,从而减弱了它们的抑制作用。该研究为钢铁/焦化行业多污染物协同控制提供理论依据和技术支撑。
更新日期:2024-04-06
中文翻译:

深入探讨MnCuTi三元催化剂同时脱除NOx和甲苯的机理
目前,以钢铁、焦化、水泥为代表的高耗能非电行业烟气排放已成为我国大气污染的主要来源。其中,挥发性有机化合物(VOC)和氮氧化物(NO)是PM和臭氧污染的主要前体。采用溶胶-凝胶法合成了一系列不同Mn-Cu摩尔比的MnCuTi三元体系催化剂,用于同时脱除NO和甲苯。结合催化性能和理化性质,结果表明MnCuTi三元催化剂具有较大的比表面积和丰富的介孔结构。锰和铜之间的协同作用促进了氧化还原反应的循环电子迁移,提高了NO还原和甲苯氧化的协同反应性能。在不同的催化剂中,1.5MCT催化剂表现出最好的协同活性,在240℃时NO转化率为87%,甲苯转化率为92%。原位DRIFTS彻底揭示了协同反应过程中的相互作用机制和中间物种的演化,发现NH和NO通过竞争吸附和硝酸盐沉积抑制了甲苯氧化。然而,当同时引入NH和NOx时,它们优先生成NHNO,从而减弱了它们的抑制作用。该研究为钢铁/焦化行业多污染物协同控制提供理论依据和技术支撑。



























 京公网安备 11010802027423号
京公网安备 11010802027423号