当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measuring mechanical cues for modeling the stromal matrix in 3D cell cultures
Soft Matter ( IF 3.4 ) Pub Date : 2024-04-08 , DOI: 10.1039/d3sm01425h Linda Srbova 1 , Ossi Arasalo 1 , Arttu J. Lehtonen 1 , Juho Pokki 1
Soft Matter ( IF 3.4 ) Pub Date : 2024-04-08 , DOI: 10.1039/d3sm01425h Linda Srbova 1 , Ossi Arasalo 1 , Arttu J. Lehtonen 1 , Juho Pokki 1
Affiliation
|
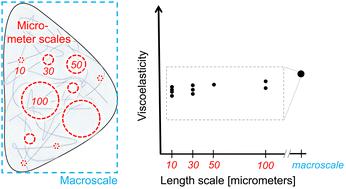
A breast-cancer tumor develops within a stroma, a tissue where a complex extracellular matrix surrounds cells, mediating the cancer progression through biomechanical and -chemical cues. Current materials partially mimic the stromal matrix in 3D cell cultures but methods for measuring the mechanical properties of the matrix at cell-relevant-length scales and stromal-stiffness levels are lacking. Here, to address this gap, we developed a characterization approach that employs probe-based microrheometry and Bayesian modeling to quantify length-scale-dependent mechanics and mechanical heterogeneity as in the stromal matrix. We examined the interpenetrating network (IPN) composed of alginate scaffolds (for adjusting mechanics) and type-1 collagen (a stromal-matrix constituent). We analyzed viscoelasticity: absolute-shear moduli (stiffness/elasticity) and phase angles (viscous and elastic characteristics). We determined the relationship between microrheometry and rheometry information. Microrheometry reveals lower stiffness at cell-relevant scales, compared to macroscale rheometry, with dependency on the length scale (10 to 100 μm). These data show increasing IPN stiffness with crosslinking until saturation (≃15 mM of Ca2+). Furthermore, we report that IPN stiffness can be adjusted by modulating collagen concentration and interconnectivity (by polymerization temperature). The IPNs are heterogeneous structurally (in SEM) and mechanically. Interestingly, increased alginate crosslinking changes IPN heterogeneity in stiffness but not in phase angle, until the saturation. In contrast, such changes are undetectable in alginate scaffolds. Our nonlinear viscoelasticity analysis at tumor-cell-exerted strains shows that only the softer IPNs stiffen with strain, like the stromal-collagen constituent. In summary, our approach can quantify the stromal-matrix-related viscoelasticity and is likely applicable to other materials in 3D culture.
中文翻译:

测量机械线索以模拟 3D 细胞培养中的基质基质
乳腺癌肿瘤在基质内发育,基质是一种复杂的细胞外基质包围细胞的组织,通过生物力学和化学线索介导癌症进展。目前的材料部分模仿 3D 细胞培养物中的基质基质,但缺乏在细胞相关长度尺度和基质硬度水平上测量基质机械性能的方法。在这里,为了解决这一差距,我们开发了一种表征方法,采用基于探针的微流变测量和贝叶斯建模来量化基质基质中的长度尺度依赖性力学和机械异质性。我们检查了由藻酸盐支架(用于调整力学)和 1 型胶原(基质基质成分)组成的互穿网络 (IPN)。我们分析了粘弹性:绝对剪切模量(刚度/弹性)和相角(粘性和弹性特性)。我们确定了微流变测量和流变测量信息之间的关系。与宏观流变测量相比,微流变测量显示细胞相关尺度的刚度较低,且取决于长度尺度(10 至 100 μm)。这些数据显示随着交联直至饱和(≃15 mM Ca 2+),IPN 刚度不断增加。此外,我们报告说,IPN 刚度可以通过调节胶原蛋白浓度和互连性(通过聚合温度)来调节。 IPNs 在结构上(在 SEM 中)和机械上是异质的。有趣的是,增加的藻酸盐交联会改变 IPN 的刚度异质性,但不会改变相角,直至饱和。相比之下,这种变化在藻酸盐支架中是无法检测到的。我们对肿瘤细胞施加的应变进行的非线性粘弹性分析表明,只有较软的 IPN 会随着应变而变硬,如基质胶原成分。总之,我们的方法可以量化基质相关的粘弹性,并且可能适用于 3D 培养中的其他材料。
更新日期:2024-04-08
中文翻译:

测量机械线索以模拟 3D 细胞培养中的基质基质
乳腺癌肿瘤在基质内发育,基质是一种复杂的细胞外基质包围细胞的组织,通过生物力学和化学线索介导癌症进展。目前的材料部分模仿 3D 细胞培养物中的基质基质,但缺乏在细胞相关长度尺度和基质硬度水平上测量基质机械性能的方法。在这里,为了解决这一差距,我们开发了一种表征方法,采用基于探针的微流变测量和贝叶斯建模来量化基质基质中的长度尺度依赖性力学和机械异质性。我们检查了由藻酸盐支架(用于调整力学)和 1 型胶原(基质基质成分)组成的互穿网络 (IPN)。我们分析了粘弹性:绝对剪切模量(刚度/弹性)和相角(粘性和弹性特性)。我们确定了微流变测量和流变测量信息之间的关系。与宏观流变测量相比,微流变测量显示细胞相关尺度的刚度较低,且取决于长度尺度(10 至 100 μm)。这些数据显示随着交联直至饱和(≃15 mM Ca 2+),IPN 刚度不断增加。此外,我们报告说,IPN 刚度可以通过调节胶原蛋白浓度和互连性(通过聚合温度)来调节。 IPNs 在结构上(在 SEM 中)和机械上是异质的。有趣的是,增加的藻酸盐交联会改变 IPN 的刚度异质性,但不会改变相角,直至饱和。相比之下,这种变化在藻酸盐支架中是无法检测到的。我们对肿瘤细胞施加的应变进行的非线性粘弹性分析表明,只有较软的 IPN 会随着应变而变硬,如基质胶原成分。总之,我们的方法可以量化基质相关的粘弹性,并且可能适用于 3D 培养中的其他材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号