当前位置:
X-MOL 学术
›
J. Diabetes
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy and safety of bexagliflozin compared with dapagliflozin as an adjunct to metformin in Chinese patients with type 2 diabetes mellitus: A 24‐week, randomized, double‐blind, active‐controlled, phase 3 trial
Journal of Diabetes ( IF 4.5 ) Pub Date : 2024-04-08 , DOI: 10.1111/1753-0407.13526 Lingding Xie 1 , Jie Han 2 , Zhifeng Cheng 3 , Dexue Liu 4 , Jie Liu 5 , Chunrong Xu 6 , Wenli Sun 7 , Qingju Li 8 , Fang Bian 9 , Wei Zhang 10 , Jinyu Chen 10 , Qian Zhu 10 , Tara K. Thurber 11 , J. Paul Lock 12 , Bo Zhang 1
Journal of Diabetes ( IF 4.5 ) Pub Date : 2024-04-08 , DOI: 10.1111/1753-0407.13526 Lingding Xie 1 , Jie Han 2 , Zhifeng Cheng 3 , Dexue Liu 4 , Jie Liu 5 , Chunrong Xu 6 , Wenli Sun 7 , Qingju Li 8 , Fang Bian 9 , Wei Zhang 10 , Jinyu Chen 10 , Qian Zhu 10 , Tara K. Thurber 11 , J. Paul Lock 12 , Bo Zhang 1
Affiliation
|
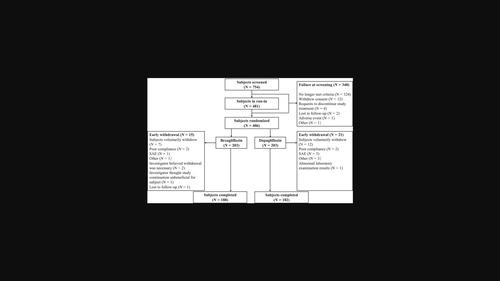
BackgroundBexagliflozin and dapagliflozin are sodium‐glucose cotransporter‐2 (SGLT2) inhibitors. No direct comparison of SGLT2 inhibitors in a randomized controlled trial has been reported to date.MethodsThis was a multicenter, randomized, double‐blind, active‐controlled trial comparing bexagliflozin to dapagliflozin for the treatment of type 2 diabetes mellitus in adults with disease inadequately controlled by metformin. Subjects (n = 406) were randomized to receive bexagliflozin (20 mg) or dapagliflozin (10 mg) plus metformin. The primary endpoint was noninferiority of bexagliflozin to dapagliflozin for the change in glycated hemoglobin (HbA1c) from baseline to week 24. Secondary endpoints included intergroup differences in fasting plasma glucose (FPG), 2‐h‐postprandial glucose (PPG), body weight, and systolic blood pressure (SBP) from baseline to week 24. The trial also evaluated the safety profiles.ResultsThe model‐adjusted mean change from baseline to week 24 HbA1c was −1.08% for bexagliflozin and −1.10% for dapagliflozin. The intergroup difference of 0.03% (95% confidence interval [CI] −0.14% to 0.19%) was below the prespecified margin of 0.4%, confirming the noninferiority of bexagliflozin. The changes from baseline in FPG, PPG, body weight, and SBP were −1.95 mmol/L, −3.24 mmol/L, −2.52 kg, and −6.4 mm Hg in the bexagliflozin arm and −1.87 mmol/L, −3.07 mmol/L, −2.22 kg, and −6.3 mm Hg in the dapagliflozin arm. Adverse events were experienced in 62.6% and 65.0% and serious adverse events affected 4.4% and 3.5% of subjects in the bexagliflozin and dapagliflozin arm, respectively.ConclusionsBexagliflozin showed nearly identical effects and a similar safety profile to dapagliflozin when used in Chinese patients on metformin.image
更新日期:2024-04-08



























 京公网安备 11010802027423号
京公网安备 11010802027423号