Adsorption ( IF 3.3 ) Pub Date : 2024-04-07 , DOI: 10.1007/s10450-024-00442-1 Débora A. S. Maia , Thalita M. Azevedo , Daniele S. Pereira , Rhuan A. M. Castro , Beatriz O. Nascimento , Enrique Rodríguez-Castellón , Moisés Bastos-Neto , Diana C. S. Azevedo
|
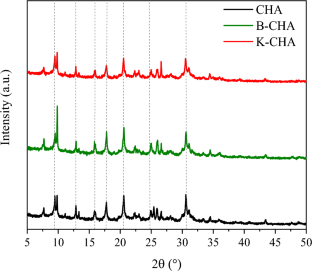
Ion exchange is the reversible exchange of ions in which there is no significant change in the solid structure. Zeolites are aluminosilicates with a defined structure, including cavities occupied by cations and water molecules, both with great freedom of movement, which makes cation exchange possible. In this study, small-pore zeolites chabazite (CHA) and clinoptilolite (CLI) were ion-exchanged with potassium. Then, the samples were characterized by N2 isotherms at 77 K, CO2 adsorption microcalorimetry at 298 K, and water vapor isotherms at 313 K. A mathematical model was applied to evaluate the adsorption kinetics for water vapor uptakes. Textural analysis showed that the ion exchange with potassium decreased the porosity of both zeolites, but CO2 microcalorimetric data showed that these samples had higher CO2 adsorption enthalpy, indicating a greater sorbate-sorbent interaction as compared to the pristine zeolites. Uptake rate curves suggest water diffusion is not appreciably altered after ion exchange. Interestingly, despite the larger size of K+ cations as compared to Na+, effective diffusion time constant is on order of magnitude larger for the potassium-loaded CLI very likely due to the leaching of other contaminants upon ion-exchange.
中文翻译:

小孔离子交换沸石对水蒸气的吸附
离子交换是离子的可逆交换,其中固体结构没有显着变化。沸石是具有确定结构的铝硅酸盐,包括被阳离子和水分子占据的空腔,两者都具有很大的运动自由度,这使得阳离子交换成为可能。在这项研究中,小孔沸石菱沸石(CHA)和斜发沸石(CLI)与钾进行离子交换。然后,通过77 K 的N 2 等温线、298 K 的CO 2吸附微量热法和313 K 的水蒸气等温线对样品进行表征。应用数学模型来评估水蒸气吸收的吸附动力学。结构分析表明,与钾的离子交换降低了两种沸石的孔隙率,但CO 2微量热数据表明,这些样品具有更高的CO 2吸附焓,表明与原始沸石相比,吸附剂-吸附剂相互作用更大。吸收率曲线表明离子交换后水扩散没有明显改变。有趣的是,尽管 K +阳离子的尺寸比 Na +更大,但负载钾的 CLI 的有效扩散时间常数很可能是由于离子交换时其他污染物的浸出所致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号