当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comprehensive Characterization of LAT1 Cholesterol-Binding Sites
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2024-04-10 , DOI: 10.1021/acs.jctc.3c01391 Keino Hutchinson 1 , Avner Schlessinger 1, 2
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2024-04-10 , DOI: 10.1021/acs.jctc.3c01391 Keino Hutchinson 1 , Avner Schlessinger 1, 2
Affiliation
|
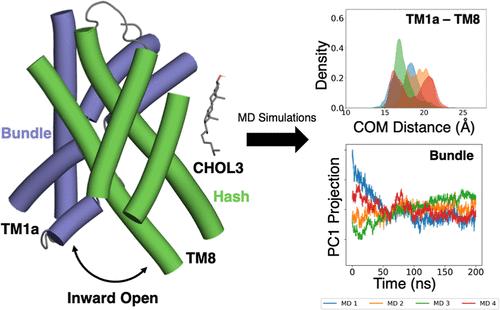
The human L-type amino acid transporter 1 (LAT1; SLC7A5), is an amino acid exchanger protein, primarily found in the blood–brain barrier, placenta, and testis, where it plays a key role in amino acid homeostasis. Cholesterol is an essential lipid that has been highlighted to play a role in regulating the activity of membrane transporters, such as LAT1, yet little is known about the molecular mechanisms driving this phenomenon. Here we perform a comprehensive computational analysis to investigate cholesterol’s role in LAT1 structure and function, focusing on four cholesterol-binding sites (CHOL1-4) identified in a recent LAT1-apo inward-open conformation cryo-EM structure. Through a series of independent molecular dynamics (MD) simulations, molecular docking, MM/GBSA free energy calculations, and other analysis tools, we explored the interactions between LAT1 and cholesterol. Our findings suggest that CHOL3 forms the most stable and favorable interactions with LAT1. Principal component analysis (PCA) and center of mass (COM) distance assessments show that CHOL3 binding stabilizes the inward-open state of LAT1 by preserving the spatial arrangement of the hash and bundle domains. Additionally, we propose an alternative cholesterol-binding site for originally assigned CHOL1. Overall, this study improves the understanding of cholesterol’s modulatory effect on LAT1 and proposes candidate sites for the discovery of future allosteric ligands with rational design.
中文翻译:

LAT1 胆固醇结合位点的综合表征
人类 L 型氨基酸转运蛋白 1(LAT1;SLC7A5)是一种氨基酸交换蛋白,主要存在于血脑屏障、胎盘和睾丸中,在氨基酸稳态中发挥着关键作用。胆固醇是一种重要的脂质,它在调节膜转运蛋白(如 LAT1)的活性方面发挥着重要作用,但人们对驱动这种现象的分子机制知之甚少。在这里,我们进行了全面的计算分析,以研究胆固醇在 LAT1 结构和功能中的作用,重点关注最近在 LAT1-apo 内向开放构象冷冻电镜结构中发现的四个胆固醇结合位点 (CHOL1-4)。通过一系列独立的分子动力学(MD)模拟、分子对接、MM/GBSA自由能计算和其他分析工具,我们探索了LAT1与胆固醇之间的相互作用。我们的研究结果表明 CHOL3 与 LAT1 形成最稳定和有利的相互作用。主成分分析 (PCA) 和质心 (COM) 距离评估表明,CHOL3 结合通过保留散列域和束域的空间排列来稳定 LAT1 的向内开放状态。此外,我们还为最初指定的 CHOL1 提出了一个替代的胆固醇结合位点。总体而言,这项研究提高了对胆固醇对 LAT1 调节作用的理解,并为通过合理设计发现未来变构配体提出了候选位点。
更新日期:2024-04-10
中文翻译:

LAT1 胆固醇结合位点的综合表征
人类 L 型氨基酸转运蛋白 1(LAT1;SLC7A5)是一种氨基酸交换蛋白,主要存在于血脑屏障、胎盘和睾丸中,在氨基酸稳态中发挥着关键作用。胆固醇是一种重要的脂质,它在调节膜转运蛋白(如 LAT1)的活性方面发挥着重要作用,但人们对驱动这种现象的分子机制知之甚少。在这里,我们进行了全面的计算分析,以研究胆固醇在 LAT1 结构和功能中的作用,重点关注最近在 LAT1-apo 内向开放构象冷冻电镜结构中发现的四个胆固醇结合位点 (CHOL1-4)。通过一系列独立的分子动力学(MD)模拟、分子对接、MM/GBSA自由能计算和其他分析工具,我们探索了LAT1与胆固醇之间的相互作用。我们的研究结果表明 CHOL3 与 LAT1 形成最稳定和有利的相互作用。主成分分析 (PCA) 和质心 (COM) 距离评估表明,CHOL3 结合通过保留散列域和束域的空间排列来稳定 LAT1 的向内开放状态。此外,我们还为最初指定的 CHOL1 提出了一个替代的胆固醇结合位点。总体而言,这项研究提高了对胆固醇对 LAT1 调节作用的理解,并为通过合理设计发现未来变构配体提出了候选位点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号