Molecular Neurobiology ( IF 5.1 ) Pub Date : 2024-04-09 , DOI: 10.1007/s12035-024-04141-4 Yujin Ahn , Ju-Hyun An , Hae-Jun Yang , Wi-Jae Lee , Sang-Hee Lee , Young-Ho Park , Jong-Hee Lee , Hong J. Lee , Seung Hwan Lee , Sun-Uk Kim
|
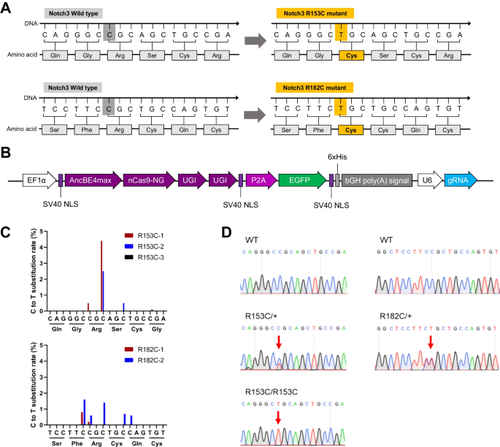
Human blood vessel organoids (hBVOs) offer a promising platform for investigating vascular diseases and identifying therapeutic targets. In this study, we focused on in vitro modeling and therapeutic target finding of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), the most common form of hereditary stroke disorder caused by mutations in the NOTCH3 gene. Despite the identification of these mutations, the underlying pathological mechanism is elusive, and effective therapeutic approaches are lacking. CADASIL primarily affects the blood vessels in the brain, leading to ischemic strokes, migraines, and dementia. By employing CRISPR/Cas9 base-editing technology, we generated human induced pluripotent stem cells (hiPSCs) carrying Notch3 mutations. These mutant hiPSCs were differentiated into hBVOs. The NOTCH3 mutated hBVOs exhibited CADASIL-like pathology, characterized by a reduced vessel diameter and degeneration of mural cells. Furthermore, we observed an accumulation of Notch3 extracellular domain (Notch3ECD), increased apoptosis, and cytoskeletal alterations in the NOTCH3 mutant hBVOs. Notably, treatment with ROCK inhibitors partially restored the disconnection between endothelial cells and mural cells in the mutant hBVOs. These findings shed light on the pathogenesis of CADASIL and highlight the potential of hBVOs for studying and developing therapeutic interventions for this debilitating human vascular disorder.
中文翻译:

通过碱基编辑生成的血管类器官和 Notch3 中含有单核苷酸变异有效地概括了 CADASIL 相关的发病机制
人类血管类器官(hBVO)为研究血管疾病和确定治疗靶点提供了一个有前景的平台。在这项研究中,我们重点关注伴有皮质下梗死和白质脑病的常染色体显性遗传性脑动脉病(CADASIL)的体外建模和治疗靶点寻找,CADASIL是由NOTCH3基因突变引起的遗传性中风疾病的最常见形式。尽管识别了这些突变,但潜在的病理机制尚不清楚,并且缺乏有效的治疗方法。 CADASIL 主要影响大脑血管,导致缺血性中风、偏头痛和痴呆。通过采用CRISPR/Cas9碱基编辑技术,我们产生了携带Notch3突变的人类诱导多能干细胞(hiPSC)。这些突变的 hiPSC 分化为 hBVO。 NOTCH3突变的hBVO表现出类似CADASIL的病理学,其特征是血管直径减小和壁细胞变性。此外,我们观察到 NOTCH3 突变 hBVO 中 Notch3 胞外结构域 (Notch3ECD) 的积累、细胞凋亡增加和细胞骨架改变。值得注意的是,用 ROCK 抑制剂治疗部分恢复了突变 hBVO 中内皮细胞和壁细胞之间的断开。这些发现揭示了 CADASIL 的发病机制,并强调了 hBVO 在研究和开发这种使人衰弱的人类血管疾病的治疗干预措施方面的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号