Journal of Fluorescence ( IF 2.7 ) Pub Date : 2024-04-09 , DOI: 10.1007/s10895-024-03689-7 Umer Sherefedin , Abebe Belay , Kusse Gudishe , Alemu Kebede , Alemayehu Getahun Kumela , Semahegn Asemare
|
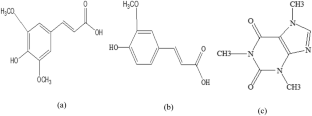
Sinapic acid (SA) and ferulic acid (FA) are bioactive compounds used in the food, pharmaceutical, and cosmetic industries due to their antioxidant properties. In this work, we studied the photophysical properties of SA and FA in different solvents and concentrations and their interactions with caffeine (CF), using ultraviolet–visible (UV–Vis), fluorescence spectroscopy and Fourier transform infrared (FTIR) spectroscopy. The findings show that the quantum yield, fluorescence lifetime, radiative decay rates, and non-radiative decay rates of SA and FA are influenced by the concentrations and solvent polarity. The interaction between SA and FA with CF was also studied using UV–Vis and fluorescence spectroscopy. The results indicate that the CF quenched the fluorescence intensity of SA and FA by static quenching due to the formation of a non-fluorescent complex. The van't Hoff equation suggests that the van der Waals forces and hydrogen bonds force were responsible for the interaction between SA and CF, as indicated by a negative change in enthalpy (\({\Delta {\text{H}}}^{{\text{o}}}\) < 0) and a negative change in entropy (\({\Delta {\text{S}}}^{{\text{o}}}\) < 0). On the other hand, the interaction between FA and CF was primarily controlled by electrostatic force, as indicated by a negative change in enthalpy (\({\Delta {\text{H}}}^{{\text{o}}}\) < 0) and a positive change in entropy (\({\Delta {\text{S}}}^{{\text{o}}}\) > 0). The negative change in Gibbs free energy (\({\Delta {\text{G}}}^{{\text{o}}}\)) indicates that both compounds underwent a spontaneous binding process.
中文翻译:

芥子酸和阿魏酸的光物理性质及其与咖啡因的结合机制
芥子酸 (SA) 和阿魏酸 (FA) 是具有抗氧化特性的生物活性化合物,可用于食品、制药和化妆品行业。在这项工作中,我们利用紫外可见光 (UV-Vis)、荧光光谱和傅里叶变换红外 (FTIR) 光谱研究了 SA 和 FA 在不同溶剂和浓度下的光物理性质及其与咖啡因 (CF) 的相互作用。研究结果表明,SA和FA的量子产率、荧光寿命、辐射衰变率和非辐射衰变率受到浓度和溶剂极性的影响。还使用紫外-可见光和荧光光谱研究了 SA 和 FA 与 CF 之间的相互作用。结果表明,CF由于形成非荧光复合物而通过静态猝灭来猝灭SA和FA的荧光强度。范特霍夫方程表明,范德华力和氢键力是 SA 和 CF 之间相互作用的原因,如焓的负变化所示 ( \({\Delta {\text{H}}}^ {{\text{o}}}\) < 0) 和熵的负变化 ( \({\Delta {\text{S}}}^{{\text{o}}}\) < 0)。另一方面,FA 和 CF 之间的相互作用主要受静电力控制,如焓的负变化所示 ( \({\Delta {\text{H}}}^{{\text{o}}} \) < 0) 和熵的正变化 ( \({\Delta {\text{S}}}^{{\text{o}}}\) > 0)。吉布斯自由能的负变化 ( \({\Delta {\text{G}}}^{{\text{o}}}\) ) 表明两种化合物都经历了自发结合过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号