当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid-Phase Synthesis of Peptidols via Reductive Cleavage Through a Benzotriazole Linker
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-04-10 , DOI: 10.1002/adsc.202400204 Szu-Hsuan Chen, Hui-Ying Chuang, Chitra Rajavel, Yi Kai Lin, Shu-Han Chang, Pei-Chen Tsai, Hui-Ting Chen, Chung-Ming Sun, Anand Selvaraj, Chai-Lin Kao
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-04-10 , DOI: 10.1002/adsc.202400204 Szu-Hsuan Chen, Hui-Ying Chuang, Chitra Rajavel, Yi Kai Lin, Shu-Han Chang, Pei-Chen Tsai, Hui-Ting Chen, Chung-Ming Sun, Anand Selvaraj, Chai-Lin Kao
|
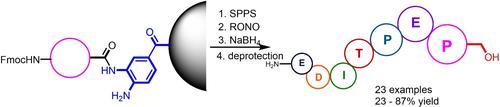
Peptidols were prepared through the reductive cleavage of benzotriazole intermediates obtained from the on‐bead activation of 3,4‐diaminobenzoyl linkers. Remarkably, this method used commercially available amino acid residues and resins without further modification. Pure peptidols were collected with only filtration in 49%–87% yield. Only the derivatives with aspartic acid as the first C‐terminal residue required sophisticated chromatography purification. Esters and carboxylic acids were identified as side products and were suppressed by additional reduction or reduction in DMF. Remarkably, the reduction of peptides with the first aspartic acid residue at the C‐terminal afforded a C‐terminal lactone, which could be reduced by deprotection at 0°C. For the synthesis of branched peptidols, the bulky wedge structures of the branched peptides resulted in low total yields, which were improved to 23%–28% by using peripheral Boc groups and SPPS on the Tental gel resin. In addition, this method gave two peptaibols, SPF‐5506‐A4 and Ac‐Gramicidin A, in 11% and 46% yields, respectively.
中文翻译:

通过苯并三唑连接体还原裂解固相合成肽醇
通过对 3,4-二氨基苯甲酰基连接体的珠上活化获得的苯并三唑中间体进行还原裂解来制备肽醇。值得注意的是,该方法使用市售的氨基酸残基和树脂,无需进一步修改。仅通过过滤即可收集纯肽,收率为 49%–87%。只有以天冬氨酸作为第一个 C 末端残基的衍生物才需要复杂的色谱纯化。酯和羧酸被确定为副产物,并通过额外减少或减少 DMF 来抑制。值得注意的是,C 末端第一个天冬氨酸残基的肽的还原提供了 C 末端内酯,可以通过在 0°C 脱保护来还原。对于支链肽的合成,支链肽庞大的楔形结构导致总产率较低,通过在 Tental 凝胶树脂上使用外周 Boc 基团和 SPPS 将总产率提高到 23%–28%。此外,该方法还得到了两种 peptaibols:SPF-5506-A4 和 Ac-Gramicidin A,产率分别为 11% 和 46%。
更新日期:2024-04-10
中文翻译:

通过苯并三唑连接体还原裂解固相合成肽醇
通过对 3,4-二氨基苯甲酰基连接体的珠上活化获得的苯并三唑中间体进行还原裂解来制备肽醇。值得注意的是,该方法使用市售的氨基酸残基和树脂,无需进一步修改。仅通过过滤即可收集纯肽,收率为 49%–87%。只有以天冬氨酸作为第一个 C 末端残基的衍生物才需要复杂的色谱纯化。酯和羧酸被确定为副产物,并通过额外减少或减少 DMF 来抑制。值得注意的是,C 末端第一个天冬氨酸残基的肽的还原提供了 C 末端内酯,可以通过在 0°C 脱保护来还原。对于支链肽的合成,支链肽庞大的楔形结构导致总产率较低,通过在 Tental 凝胶树脂上使用外周 Boc 基团和 SPPS 将总产率提高到 23%–28%。此外,该方法还得到了两种 peptaibols:SPF-5506-A4 和 Ac-Gramicidin A,产率分别为 11% 和 46%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号