Synthesis ( IF 2.6 ) Pub Date : 2024-04-10 , DOI: 10.1055/a-2295-8544 Alexander S. Fisyuk , Anna L. Samsonenko , Anastasia S. Kostyuchenko , Tatyana Yu. Zheleznova , Vladislav Yu. Shuvalov , Evgenii B. Uliankin , Anton L. Shatsauskas 1
|
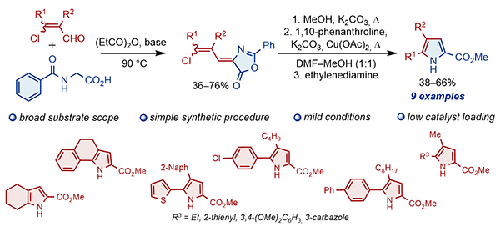
The Vilsmeier–Haack reaction of ketones with DMF and POCl3 produced 3-chloroacrylcarbaldehydes, which were converted into the corresponding (Z)-4-[(Z)- or (Z)-4-[(E)-3-chloroallylidene)-2-phenyloxazol-5(4H)-ones] (azlactones) or their isomeric mixtures when heated with hippuric acid in propionic anhydride. It was shown that the alcoholysis products of these compounds, resulting from the opening of the oxazolone ring, undergo copper-catalyzed intramolecular cross-coupling reactions with the formation of methyl 1H-pyrrole-2-carboxylates. Thus, a one-pot method was developed for the synthesis of methyl 1H-pyrrole-2-carboxylates starting from 4-(3-chloroallylidene)-2-phenyloxazol-5(4H)-ones.
中文翻译:

由3-氯丙烯醛和马尿酸合成4,5-二取代1H-吡咯-2-甲酸甲酯
酮与 DMF 和 POCl 3 的 Vilsmeier-Haack 反应生成3-氯丙烯酰甲醛,将其转化为相应的 ( Z ) -4-[( Z ) -或 ( Z ) -4-[( E ) -3-氯烯丙基 ) -2-苯基恶唑-5(4H ) -酮](吖内酯)或其异构体混合物,与丙酸酐中的马尿酸一起加热。结果表明,这些化合物的醇解产物是由恶唑酮环打开而产生的,会发生铜催化的分子内交叉偶联反应,形成1H-吡咯-2-羧酸甲酯。因此,开发了一种以4-(3-氯烯丙基)-2-苯基恶唑-5(4H)-酮为原料合成1H-吡咯-2-甲酸甲酯的一锅法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号