当前位置:
X-MOL 学术
›
J. Am. Soc. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improved Detection of Tryptic Peptides from Tissue Sections Using Desorption Electrospray Ionization Mass Spectrometry Imaging
Journal of the American Society for Mass Spectrometry ( IF 3.2 ) Pub Date : 2024-04-11 , DOI: 10.1021/jasms.4c00006 Heather Bottomley 1 , Jonathan Phillips 1 , Philippa Hart 2
Journal of the American Society for Mass Spectrometry ( IF 3.2 ) Pub Date : 2024-04-11 , DOI: 10.1021/jasms.4c00006 Heather Bottomley 1 , Jonathan Phillips 1 , Philippa Hart 2
Affiliation
|
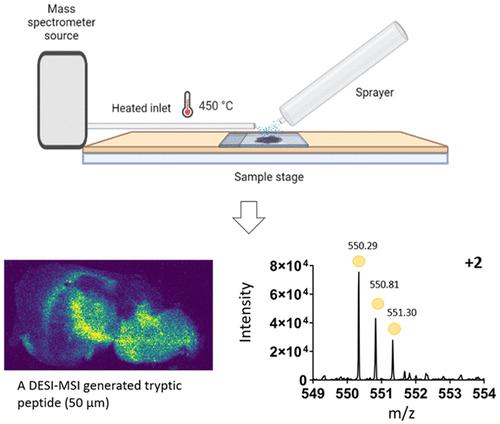
DESI-MSI is an ambient ionization technique used frequently for the detection of lipids, small molecules, and drug targets. Until recently, DESI had only limited use for the detection of proteins and peptides due to the setup and needs around deconvolution of data resulting in a small number of species being detected at lower spatial resolution. There are known differences in the ion species detected using DESI and MALDI for nonpeptide molecules, and here, we identify that this extends to proteomic species. DESI MS images were obtained for tissue sections of mouse and rat brain using a precommercial heated inlet (approximately 450 °C) to the mass spectrometer. Ion mobility separation resolved spectral overlap of peptide ions and significantly improved the detection of multiply charged species. The images acquired were of pixel size 100 μm (rat brain) and 50 μm (mouse brain), respectively. Observed tryptic peptides were filtered against proteomic target lists, generated by LC–MS, enabling tentative protein assignment for each peptide ion image. Precise localizations of peptide ions identified by DESI and MALDI were found to be comparable. Some spatially localized peptides ions were observed in DESI that were not found in the MALDI replicates, typically, multiply charged species with a low mass to charge ratio. This method demonstrates the potential of DESI-MSI to detect large numbers of tryptic peptides from tissue sections with enhanced spatial resolution when compared to previous DESI-MSI studies.
中文翻译:

使用解吸电喷雾电离质谱成像改进组织切片中胰蛋白酶肽的检测
DESI-MSI 是一种常用于检测脂质、小分子和药物靶标的环境电离技术。直到最近,由于数据反卷积的设置和需求,导致在较低空间分辨率下检测到少量物种,DESI 在蛋白质和肽检测方面的用途有限。对于非肽分子,使用 DESI 和 MALDI 检测到的离子种类存在已知差异,在这里,我们确定这种差异延伸到了蛋白质组种类。使用质谱仪的预商业加热入口(约 450 °C)获得小鼠和大鼠大脑组织切片的 DESI MS 图像。离子淌度分离解决了肽离子的光谱重叠问题,并显着改善了多电荷物质的检测。获取的图像像素大小分别为 100 μm(大鼠脑)和 50 μm(小鼠脑)。根据 LC-MS 生成的蛋白质组目标列表过滤观察到的胰蛋白酶肽,从而为每个肽离子图像进行尝试性蛋白质分配。发现 DESI 和 MALDI 识别的肽离子的精确定位具有可比性。在 DESI 中观察到一些空间定位的肽离子,而在 MALDI 重复中未发现这些离子,通常是具有低质荷比的多电荷物质。与之前的 DESI-MSI 研究相比,该方法证明了 DESI-MSI 从组织切片中检测大量胰蛋白酶肽的潜力,并且具有增强的空间分辨率。
更新日期:2024-04-11
中文翻译:

使用解吸电喷雾电离质谱成像改进组织切片中胰蛋白酶肽的检测
DESI-MSI 是一种常用于检测脂质、小分子和药物靶标的环境电离技术。直到最近,由于数据反卷积的设置和需求,导致在较低空间分辨率下检测到少量物种,DESI 在蛋白质和肽检测方面的用途有限。对于非肽分子,使用 DESI 和 MALDI 检测到的离子种类存在已知差异,在这里,我们确定这种差异延伸到了蛋白质组种类。使用质谱仪的预商业加热入口(约 450 °C)获得小鼠和大鼠大脑组织切片的 DESI MS 图像。离子淌度分离解决了肽离子的光谱重叠问题,并显着改善了多电荷物质的检测。获取的图像像素大小分别为 100 μm(大鼠脑)和 50 μm(小鼠脑)。根据 LC-MS 生成的蛋白质组目标列表过滤观察到的胰蛋白酶肽,从而为每个肽离子图像进行尝试性蛋白质分配。发现 DESI 和 MALDI 识别的肽离子的精确定位具有可比性。在 DESI 中观察到一些空间定位的肽离子,而在 MALDI 重复中未发现这些离子,通常是具有低质荷比的多电荷物质。与之前的 DESI-MSI 研究相比,该方法证明了 DESI-MSI 从组织切片中检测大量胰蛋白酶肽的潜力,并且具有增强的空间分辨率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号