当前位置:
X-MOL 学术
›
Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational investigation on adsorption and activation of atmospheric pollutants CO, NO and SO on small cobalt clusters
Chemical Physics ( IF 2.3 ) Pub Date : 2024-04-07 , DOI: 10.1016/j.chemphys.2024.112291 Muhammed Shabeeb , Surajit Maity
Chemical Physics ( IF 2.3 ) Pub Date : 2024-04-07 , DOI: 10.1016/j.chemphys.2024.112291 Muhammed Shabeeb , Surajit Maity
|
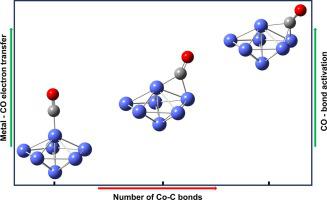
The adsorption of CO, NO and SO on cobalt clusters (Co) were investigated using density functional theory. The adsorption energy supports efficient chemisorption of greenhouse gases on the cobalt clusters, with CO and NO forming one to three CoC and CoN bonds, respectively, the first being the most stable. The SO formed bidentate complexes with CoS and CoO bonds in CoSO structures, displaying notably high adsorption energy. The interactions between Co clusters and gas molecules (G) result in weakened bonds of CO, NO, and SO, evident through increased bond lengths, red-shifted frequencies, and lowered local vibrational force constants in CoG complexes. The results with bond weakening and charge transfer from metal to gas molecules suggest strong catalytic potential for small cobalt clusters in activating gas molecules. The current research findings hold significance in the quest for efficient catalytic processes to capture and recycle gaseous pollutants, contributing to a sustainable future.
中文翻译:

小钴团簇吸附和活化大气污染物CO、NO和SO的计算研究
利用密度泛函理论研究了钴簇 (Co) 上 CO、NO 和 SO 的吸附。吸附能支持温室气体在钴簇上的有效化学吸附,CO 和 NO 分别形成一到三个 CoC 和 CoN 键,第一个键最稳定。 SO与CoSO结构中的CoS和CoO键形成双齿配合物,表现出极高的吸附能。 Co团簇和气体分子 (G) 之间的相互作用导致 CO、NO 和 SO 的键减弱,这通过 CoG 复合物中键长的增加、红移频率和降低的局部振动力常数来明显体现。键弱化和从金属到气体分子的电荷转移的结果表明,小钴簇在活化气体分子方面具有很强的催化潜力。目前的研究结果对于寻求有效的催化过程来捕获和回收气态污染物具有重要意义,从而为可持续的未来做出贡献。
更新日期:2024-04-07
中文翻译:

小钴团簇吸附和活化大气污染物CO、NO和SO的计算研究
利用密度泛函理论研究了钴簇 (Co) 上 CO、NO 和 SO 的吸附。吸附能支持温室气体在钴簇上的有效化学吸附,CO 和 NO 分别形成一到三个 CoC 和 CoN 键,第一个键最稳定。 SO与CoSO结构中的CoS和CoO键形成双齿配合物,表现出极高的吸附能。 Co团簇和气体分子 (G) 之间的相互作用导致 CO、NO 和 SO 的键减弱,这通过 CoG 复合物中键长的增加、红移频率和降低的局部振动力常数来明显体现。键弱化和从金属到气体分子的电荷转移的结果表明,小钴簇在活化气体分子方面具有很强的催化潜力。目前的研究结果对于寻求有效的催化过程来捕获和回收气态污染物具有重要意义,从而为可持续的未来做出贡献。



























 京公网安备 11010802027423号
京公网安备 11010802027423号