当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sustainable click reactions: Use of greener reaction media in the synthesis of 1,2,3-triazoles
Tetrahedron ( IF 2.1 ) Pub Date : 2024-03-31 , DOI: 10.1016/j.tet.2024.133964 Luan A. Martinho , Carlos Kleber Z. Andrade
Tetrahedron ( IF 2.1 ) Pub Date : 2024-03-31 , DOI: 10.1016/j.tet.2024.133964 Luan A. Martinho , Carlos Kleber Z. Andrade
|
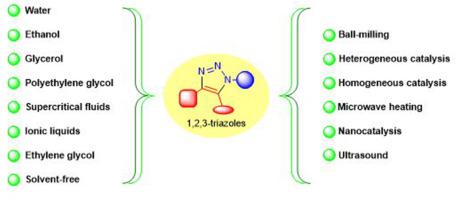
Heterocyclic compounds are widely found in nature, more specifically, five- or six-membered rings nitrogen heterocycles are important in the synthesis of biologically active compounds. An essential class of nitrogen heterocyclic substances are the 1,2,3-triazoles, which contain a five-membered ring embedding three contiguous nitrogen atoms followed by two carbon atoms. These heterocyclic compounds exhibit a wide range of biological activities with important industrial applications. The original method for 1,2,3-triazoles synthesis is the Huisgen 1,3-dipolar cycloaddition of azides and alkynes, which was popularized by Meldal and Sharpless (Nobel Prize in chemistry awardees in 2022) with the use of copper catalysts. This method is commonly called copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and belongs to a class of reactions known as “click” chemistry, which emphasizes mild reaction conditions with high yields, regiospecificity, and stereospecificity. Over the last decades, significant advances in chemical processes have been made applying the twelve principles of Green Chemistry. Not surprisingly, many environmentally friendly methodologies have been reported in “click” chemistry. This review focused on recent articles (last 10 years) that used greener reaction media (water, ethanol, glycerol, PEG, supercritical fluids, EG, ionic liquids, and solvent-free processes) to synthesize 1,2,3-triazoles. Nanocatalysis and mechanochemistry have also been addressed.
中文翻译:

可持续点击反应:在 1,2,3-三唑合成中使用更绿色的反应介质
杂环化合物广泛存在于自然界中,更具体地说,五元或六元环氮杂环在生物活性化合物的合成中具有重要意义。一类重要的氮杂环物质是 1,2,3-三唑,它包含一个五元环,其中嵌入三个连续的氮原子,然后是两个碳原子。这些杂环化合物表现出广泛的生物活性,具有重要的工业应用。 1,2,3-三唑的原始合成方法是叠氮化物和炔烃的Huisgen 1,3-偶极环加成反应,该方法由2022年诺贝尔化学奖获得者Meldal和Sharpless使用铜催化剂推广。该方法通常称为铜(I)催化叠氮化物-炔环加成(CuAAC),属于一类被称为“点击”化学的反应,强调温和的反应条件,具有高产率、区域专一性和立体专一性。在过去的几十年中,应用绿色化学的十二条原则在化学过程中取得了重大进展。毫不奇怪,“点击”化学中已经报道了许多环境友好的方法。本综述重点关注最近(过去 10 年)使用更环保的反应介质(水、乙醇、甘油、PEG、超临界流体、EG、离子液体和无溶剂工艺)合成 1,2,3-三唑的文章。纳米催化和机械化学也得到了解决。
更新日期:2024-03-31
中文翻译:

可持续点击反应:在 1,2,3-三唑合成中使用更绿色的反应介质
杂环化合物广泛存在于自然界中,更具体地说,五元或六元环氮杂环在生物活性化合物的合成中具有重要意义。一类重要的氮杂环物质是 1,2,3-三唑,它包含一个五元环,其中嵌入三个连续的氮原子,然后是两个碳原子。这些杂环化合物表现出广泛的生物活性,具有重要的工业应用。 1,2,3-三唑的原始合成方法是叠氮化物和炔烃的Huisgen 1,3-偶极环加成反应,该方法由2022年诺贝尔化学奖获得者Meldal和Sharpless使用铜催化剂推广。该方法通常称为铜(I)催化叠氮化物-炔环加成(CuAAC),属于一类被称为“点击”化学的反应,强调温和的反应条件,具有高产率、区域专一性和立体专一性。在过去的几十年中,应用绿色化学的十二条原则在化学过程中取得了重大进展。毫不奇怪,“点击”化学中已经报道了许多环境友好的方法。本综述重点关注最近(过去 10 年)使用更环保的反应介质(水、乙醇、甘油、PEG、超临界流体、EG、离子液体和无溶剂工艺)合成 1,2,3-三唑的文章。纳米催化和机械化学也得到了解决。



























 京公网安备 11010802027423号
京公网安备 11010802027423号