当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Homoallylamines Enabled by Cobalt or Palladium Catalyzed Allylic Substitution of Azaarylmethylamines
Organic Letters ( IF 5.2 ) Pub Date : 2024-04-11 , DOI: 10.1021/acs.orglett.4c00551 Jiali Zheng 1 , Rui Hua 1 , Yan-En Wang 2 , Tingzhi Lin 1 , Mingjie Ou 1 , Yu Wu 1 , En-hao Shi 1 , Jing He 1 , Dan Xiong 1 , Jianyou Mao 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-04-11 , DOI: 10.1021/acs.orglett.4c00551 Jiali Zheng 1 , Rui Hua 1 , Yan-En Wang 2 , Tingzhi Lin 1 , Mingjie Ou 1 , Yu Wu 1 , En-hao Shi 1 , Jing He 1 , Dan Xiong 1 , Jianyou Mao 1
Affiliation
|
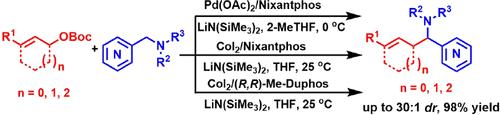
Pd(OAc)2/Nixantphos or CoI2/Nixantphos catalyzed allylic substitutions with weakly acidic C(sp)3–H bonds of azaarylmethylamines are described. This method facilitates access to various kinds of heteroaryl rings containing homoallylamines (39 examples, 30–98% yields) with excellent functional group tolerance and diastereoselectivity. Compared with the Pd/Nixantphos complex, the Co/Nixantphos catalysis could obtain the cyclic products with good to excellent diastereoselectivities. Importantly, the CoI2/(R,R)-Me-Duphos catalyzed reactions exhibit moderate enantioselectivity. Additionally, the scalability of this transformation is successfully demonstrated.
中文翻译:

钴或钯催化氮杂芳基甲基胺的烯丙基取代合成高烯丙胺
描述了Pd(OAc) 2 /Nixantphos 或 CoI 2 /Nixantphos 催化的氮杂芳基甲基胺的弱酸性 C(sp) 3 –H 键的烯丙基取代。该方法有利于获得各种含有高烯丙胺的杂芳环(39 个例子,产率 30-98%),具有优异的官能团耐受性和非对映选择性。与Pd/Nixantphos配合物相比,Co/Nixantphos催化可以获得具有良好至优异非对映选择性的环状产物。重要的是,CoI 2 /( R,R )-Me-Duphos 催化反应表现出中等的对映选择性。此外,这种转变的可扩展性也得到了成功的证明。
更新日期:2024-04-11
中文翻译:

钴或钯催化氮杂芳基甲基胺的烯丙基取代合成高烯丙胺
描述了Pd(OAc) 2 /Nixantphos 或 CoI 2 /Nixantphos 催化的氮杂芳基甲基胺的弱酸性 C(sp) 3 –H 键的烯丙基取代。该方法有利于获得各种含有高烯丙胺的杂芳环(39 个例子,产率 30-98%),具有优异的官能团耐受性和非对映选择性。与Pd/Nixantphos配合物相比,Co/Nixantphos催化可以获得具有良好至优异非对映选择性的环状产物。重要的是,CoI 2 /( R,R )-Me-Duphos 催化反应表现出中等的对映选择性。此外,这种转变的可扩展性也得到了成功的证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号