当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic, electrochemical and spectral characterization of bacterial and archaeal rusticyanins; unexpected stability issues and consequences for applications in biotechnology
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2024-03-25 , DOI: 10.1016/j.jinorgbio.2024.112539 Liam A. Wilson , Jamie N. Melville , Marcelo M. Pedroso , Stefan Krco , Robert Hoelzle , Julian Zaugg , Gordon Southam , Bernardino Virdis , Paul Evans , Jenna Supper , Jeffrey R. Harmer , Gene Tyson , Alice Clark , Gerhard Schenk , Paul V. Bernhardt
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2024-03-25 , DOI: 10.1016/j.jinorgbio.2024.112539 Liam A. Wilson , Jamie N. Melville , Marcelo M. Pedroso , Stefan Krco , Robert Hoelzle , Julian Zaugg , Gordon Southam , Bernardino Virdis , Paul Evans , Jenna Supper , Jeffrey R. Harmer , Gene Tyson , Alice Clark , Gerhard Schenk , Paul V. Bernhardt
|
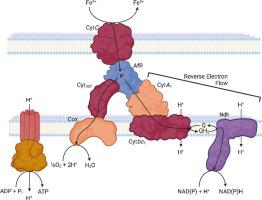
Motivated by the ambition to establish an enzyme-driven bioleaching pathway for copper extraction, properties of the Type-1 copper protein rusticyanin from (AfR) were compared with those from an ancestral form of this enzyme (N0) and an archaeal enzyme identified in (FaR). While both N0 and FaR show redox potentials similar to that of AfR their electron transport rates were significantly slower. The lack of a correlation between the redox potentials and electron transfer rates indicates that AfR and its associated electron transfer chain evolved to specifically facilitate the efficient conversion of the energy of iron oxidation to ATP formation. In this pathway is not as efficient unless it is up-regulated by an as of yet unknown mechanism. In addition, while the electrochemical properties of AfR were consistent with previous data, previously unreported behavior was found leading to a form that is associated with a partially unfolded form of the protein. The cyclic voltammetry (CV) response of AfR immobilized onto an electrode showed limited stability, which may be connected to the presence of the partially unfolded state of this protein. Insights gained in this study may thus inform the engineering of optimized rusticyanin variants for bioleaching processes as well as enzyme-catalyzed solubilization of copper-containing ores such as chalcopyrite.
中文翻译:

细菌和古细菌锈花苷的动力学、电化学和光谱表征;意外的稳定性问题及其对生物技术应用的影响
出于建立用于铜提取的酶驱动生物浸出途径的雄心,将来自 (AfR) 的 1 型铜蛋白 rusticyanin 的特性与该酶的祖先形式 (N0) 以及 (远的)。虽然 N0 和 FaR 的氧化还原电位与 AfR 相似,但它们的电子传输速率明显较慢。氧化还原电位和电子转移速率之间缺乏相关性表明 AfR 及其相关电子转移链的进化专门促进铁氧化能量有效转化为 ATP 形成。在该途径中,除非通过迄今为止未知的机制上调,否则效率不高。此外,虽然 AfR 的电化学性质与之前的数据一致,但发现之前未报道的行为导致了与该蛋白质的部分未折叠形式相关的形式。固定在电极上的 AfR 的循环伏安 (CV) 响应显示出有限的稳定性,这可能与该蛋白质部分未折叠状态的存在有关。因此,本研究中获得的见解可以为生物浸出过程以及含铜矿石(例如黄铜矿)的酶催化溶解的优化锈花青变体的工程设计提供信息。
更新日期:2024-03-25
中文翻译:

细菌和古细菌锈花苷的动力学、电化学和光谱表征;意外的稳定性问题及其对生物技术应用的影响
出于建立用于铜提取的酶驱动生物浸出途径的雄心,将来自 (AfR) 的 1 型铜蛋白 rusticyanin 的特性与该酶的祖先形式 (N0) 以及 (远的)。虽然 N0 和 FaR 的氧化还原电位与 AfR 相似,但它们的电子传输速率明显较慢。氧化还原电位和电子转移速率之间缺乏相关性表明 AfR 及其相关电子转移链的进化专门促进铁氧化能量有效转化为 ATP 形成。在该途径中,除非通过迄今为止未知的机制上调,否则效率不高。此外,虽然 AfR 的电化学性质与之前的数据一致,但发现之前未报道的行为导致了与该蛋白质的部分未折叠形式相关的形式。固定在电极上的 AfR 的循环伏安 (CV) 响应显示出有限的稳定性,这可能与该蛋白质部分未折叠状态的存在有关。因此,本研究中获得的见解可以为生物浸出过程以及含铜矿石(例如黄铜矿)的酶催化溶解的优化锈花青变体的工程设计提供信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号