当前位置:
X-MOL 学术
›
Ultrason. Sonochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Soybean isolate protein complexes with different concentrations of inulin by ultrasound treatment: Structural and functional properties
Ultrasonics Sonochemistry ( IF 8.4 ) Pub Date : 2024-03-30 , DOI: 10.1016/j.ultsonch.2024.106864 Mengmeng Wang , Sai Yang , Na Sun , Tingting Zhu , Ziteng Lian , Shicheng Dai , Jing Xu , Xiaohong Tong , Huan Wang , Lianzhou Jiang
Ultrasonics Sonochemistry ( IF 8.4 ) Pub Date : 2024-03-30 , DOI: 10.1016/j.ultsonch.2024.106864 Mengmeng Wang , Sai Yang , Na Sun , Tingting Zhu , Ziteng Lian , Shicheng Dai , Jing Xu , Xiaohong Tong , Huan Wang , Lianzhou Jiang
|
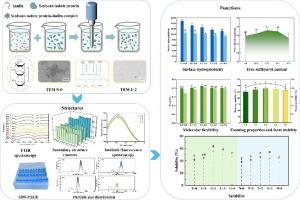
The effects of ultrasound and different inulin (INU) concentrations (0, 10, 20, 30, and 40 mg/mL) on the structural and functional properties of soybean isolate protein (SPI)-INU complexes were hereby investigated. Fourier transform infrared spectroscopy showed that SPI was bound to INU via hydrogen bonding. All samples showed a decreasing and then increasing trend of α-helix content with increasing INU concentration. SPI-INU complexes by ultrasound with an INU concentration of 20 mg/mL (U-2) had the lowest content of α-helix, the highest content of random coils and the greatest flexibility, indicating the proteins were most tightly bound to INU in U-2. Both UV spectroscopy and intrinsic fluorescence spectroscopy indicated that it was hydrophobic interactions between INU and SPI. The addition of INU prevented the exposure of tryptophan and tyrosine residues to form a more compact tertiary structure compared to SPI alone, and ultrasound caused further unfolding of the structure of SPI. This indicated that the combined effect of ultrasound and INU concentration significantly altered the tertiary structure of SPI. SDS-PAGE and Native-PAGE displayed the formation of complexes through non-covalent interactions between SPI and INU. The ζ-potential and particle size of U-2 were minimized to as low as −34.94 mV and 110 nm, respectively. Additionally, the flexibility, free sulfhydryl groups, solubility, emulsifying and foaming properties of the samples were improved, with the best results for U-2, respectively 0.25, 3.51 μmoL/g, 55.51 %, 269.91 %, 25.90 %, 137.66 % and 136.33 %. Overall, this work provides a theoretical basis for improving the functional properties of plant proteins.
中文翻译:

通过超声波处理与不同浓度菊粉的大豆分离蛋白复合物:结构和功能特性
本文研究了超声波和不同菊粉 (INU) 浓度(0、10、20、30 和 40 mg/mL)对大豆分离蛋白 (SPI)-INU 复合物结构和功能特性的影响。傅里叶变换红外光谱表明SPI通过氢键与INU结合。随着INU浓度的增加,所有样品的α-螺旋含量均呈现先减少后增加的趋势。超声检测INU浓度为20 mg/mL(U-2)的SPI-INU复合物,α螺旋含量最低,无规卷曲含量最高,柔韧性最大,表明蛋白与INU结合最紧密。 U-2。紫外光谱和本征荧光光谱均表明INU和SPI之间存在疏水相互作用。与单独的SPI相比,INU的添加阻止了色氨酸和酪氨酸残基的暴露,形成更致密的三级结构,并且超声引起SPI结构的进一步展开。这表明超声和INU浓度的联合作用显着改变了SPI的三级结构。 SDS-PAGE 和 Native-PAGE 显示通过 SPI 和 INU 之间的非共价相互作用形成复合物。 U-2 的 z 电位和粒径分别最小化至-34.94 mV 和 110 nm。此外,样品的柔韧性、游离巯基、溶解性、乳化性和发泡性均得到改善,其中U-2效果最好,分别为0.25、3.51 μmol/g、55.51 %、269.91 %、25.90 %、137.66 %和136.33%。总体而言,这项工作为改善植物蛋白的功能特性提供了理论基础。
更新日期:2024-03-30
中文翻译:

通过超声波处理与不同浓度菊粉的大豆分离蛋白复合物:结构和功能特性
本文研究了超声波和不同菊粉 (INU) 浓度(0、10、20、30 和 40 mg/mL)对大豆分离蛋白 (SPI)-INU 复合物结构和功能特性的影响。傅里叶变换红外光谱表明SPI通过氢键与INU结合。随着INU浓度的增加,所有样品的α-螺旋含量均呈现先减少后增加的趋势。超声检测INU浓度为20 mg/mL(U-2)的SPI-INU复合物,α螺旋含量最低,无规卷曲含量最高,柔韧性最大,表明蛋白与INU结合最紧密。 U-2。紫外光谱和本征荧光光谱均表明INU和SPI之间存在疏水相互作用。与单独的SPI相比,INU的添加阻止了色氨酸和酪氨酸残基的暴露,形成更致密的三级结构,并且超声引起SPI结构的进一步展开。这表明超声和INU浓度的联合作用显着改变了SPI的三级结构。 SDS-PAGE 和 Native-PAGE 显示通过 SPI 和 INU 之间的非共价相互作用形成复合物。 U-2 的 z 电位和粒径分别最小化至-34.94 mV 和 110 nm。此外,样品的柔韧性、游离巯基、溶解性、乳化性和发泡性均得到改善,其中U-2效果最好,分别为0.25、3.51 μmol/g、55.51 %、269.91 %、25.90 %、137.66 %和136.33%。总体而言,这项工作为改善植物蛋白的功能特性提供了理论基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号