当前位置:
X-MOL 学术
›
Thorac. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Real‐world evidence of efficacy of pembrolizumab plus chemotherapy and nivolumab plus ipilimumab plus chemotherapy as initial treatment for advanced non‐small cell lung cancer
Thoracic Cancer ( IF 2.9 ) Pub Date : 2024-04-11 , DOI: 10.1111/1759-7714.15304 Ayami Kaneko 1 , Nobuaki Kobayashi 1 , Kenji Miura 2 , Hiromi Matsumoto 1 , Kohei Somekawa 1 , Tomofumi Hirose 3 , Yukihito Kajita 3 , Anna Tanaka 3 , Shuhei Teranishi 3 , Yu Sairenji 2 , Hidetoshi Kawashima 4 , Kentaro Yumoto 5 , Toshinori Tsukahara 6 , Nobuhiko Fukuda 7 , Ryuichi Nishihira 4 , Keisuke Watanabe 1 , Nobuyuki Horita 1 , Yu Hara 1 , Makoto Kudo 3 , Naoki Miyazawa 8 , Takeshi Kaneko 1
Thoracic Cancer ( IF 2.9 ) Pub Date : 2024-04-11 , DOI: 10.1111/1759-7714.15304 Ayami Kaneko 1 , Nobuaki Kobayashi 1 , Kenji Miura 2 , Hiromi Matsumoto 1 , Kohei Somekawa 1 , Tomofumi Hirose 3 , Yukihito Kajita 3 , Anna Tanaka 3 , Shuhei Teranishi 3 , Yu Sairenji 2 , Hidetoshi Kawashima 4 , Kentaro Yumoto 5 , Toshinori Tsukahara 6 , Nobuhiko Fukuda 7 , Ryuichi Nishihira 4 , Keisuke Watanabe 1 , Nobuyuki Horita 1 , Yu Hara 1 , Makoto Kudo 3 , Naoki Miyazawa 8 , Takeshi Kaneko 1
Affiliation
|
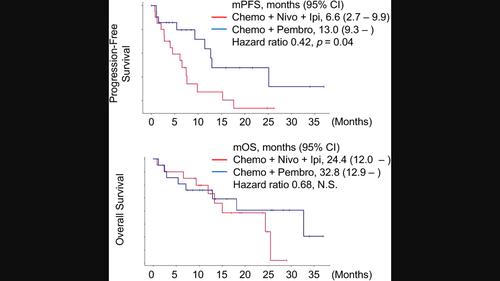
BackgroundFor advanced non‐small cell lung cancer (NSCLC), combination therapies including a PD‐1 inhibitor plus chemotherapy or a PD‐1 inhibitor, CTLA‐4 inhibitor, and chemotherapy are standard first‐line options. However, data directly comparing these regimens are lacking. This study compared the efficacy of pembrolizumab plus chemotherapy (CP) against nivolumab plus ipilimumab and chemotherapy (CNI) in a real‐world setting.MethodsIn this multicenter retrospective study, we compared the efficacy and safety of CP and CNI as first‐line therapies in 182 patients with stage IIIB–IV NSCLC. Primary outcomes were overall survival (OS) and progression‐free survival (PFS), while secondary outcomes included the response rate (RR) and safety profiles. Kaplan–Meier survival curves and Cox proportional hazards models were utilized for data analysis, adjusting for confounding factors such as age, gender, and PD‐L1 expression.ResultsIn this study, 160 patients received CP, while 22 received CNI. The CP group was associated with significantly better PFS than the CNI group (median 11.7 vs. 6.6 months, HR 0.56, p = 0.03). This PFS advantage persisted after propensity score matching to adjust for imbalances. No significant OS differences were observed. Grade 3–4 adverse events occurred comparably, but immune‐related adverse events were numerically more frequent in the CNI group.ConclusionsIn real‐world practice, CP demonstrated superior PFS compared with CNI. These findings can inform treatment selection in advanced NSCLC.
中文翻译:

帕博利珠单抗联合化疗和纳武单抗联合伊匹单抗联合化疗作为晚期非小细胞肺癌初始治疗的疗效的真实世界证据
背景对于晚期非小细胞肺癌(NSCLC),包括PD-1抑制剂加化疗或PD-1抑制剂、CTLA-4抑制剂和化疗在内的联合疗法是标准的一线选择。然而,缺乏直接比较这些方案的数据。本研究在现实环境中比较了派姆单抗联合化疗 (CP) 与纳武单抗联合伊匹单抗联合化疗 (CNI) 的疗效。方法在这项多中心回顾性研究中,我们比较了 CP 和 CNI 作为一线疗法的疗效和安全性。 182 名 IIIB-IV 期 NSCLC 患者。主要结局是总生存期(OS)和无进展生存期(PFS),次要结局包括缓解率(RR)和安全性。利用 Kaplan-Meier 生存曲线和 Cox 比例风险模型进行数据分析,调整年龄、性别和 PD-L1 表达等混杂因素。结果在本研究中,160 名患者接受了 CP,22 名患者接受了 CNI。 CP 组的 PFS 显着优于 CNI 组(中位 11.7 个月与 6.6 个月,HR 0.56,p = 0.03)。在倾向评分匹配以调整不平衡之后,这种 PFS 优势仍然存在。没有观察到显着的 OS 差异。 3-4 级不良事件的发生率相当,但 CNI 组中免疫相关不良事件的发生频率更高。结论在现实世界的实践中,CP 表现出优于 CNI 的 PFS。这些发现可以为晚期非小细胞肺癌的治疗选择提供信息。
更新日期:2024-04-11
中文翻译:

帕博利珠单抗联合化疗和纳武单抗联合伊匹单抗联合化疗作为晚期非小细胞肺癌初始治疗的疗效的真实世界证据
背景对于晚期非小细胞肺癌(NSCLC),包括PD-1抑制剂加化疗或PD-1抑制剂、CTLA-4抑制剂和化疗在内的联合疗法是标准的一线选择。然而,缺乏直接比较这些方案的数据。本研究在现实环境中比较了派姆单抗联合化疗 (CP) 与纳武单抗联合伊匹单抗联合化疗 (CNI) 的疗效。方法在这项多中心回顾性研究中,我们比较了 CP 和 CNI 作为一线疗法的疗效和安全性。 182 名 IIIB-IV 期 NSCLC 患者。主要结局是总生存期(OS)和无进展生存期(PFS),次要结局包括缓解率(RR)和安全性。利用 Kaplan-Meier 生存曲线和 Cox 比例风险模型进行数据分析,调整年龄、性别和 PD-L1 表达等混杂因素。结果在本研究中,160 名患者接受了 CP,22 名患者接受了 CNI。 CP 组的 PFS 显着优于 CNI 组(中位 11.7 个月与 6.6 个月,HR 0.56,



























 京公网安备 11010802027423号
京公网安备 11010802027423号