当前位置:
X-MOL 学术
›
CNS Neurosci. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deep‐targeted gene sequencing reveals ARID1A mutation as an important driver of glioblastoma
CNS Neuroscience & Therapeutics ( IF 5.5 ) Pub Date : 2024-04-11 , DOI: 10.1111/cns.14698 Menglin Xiao 1, 2 , Xiaoteng Cui 3 , Can Xu 1, 2 , Lei Xin 1, 2 , Jixing Zhao 3 , Shixue Yang 3 , Biao Hong 3 , Yanli Tan 4, 5 , Jie Zhang 5 , Xiang Li 5 , Jie Li 6 , Chunsheng Kang 3 , Chuan Fang 1, 2
CNS Neuroscience & Therapeutics ( IF 5.5 ) Pub Date : 2024-04-11 , DOI: 10.1111/cns.14698 Menglin Xiao 1, 2 , Xiaoteng Cui 3 , Can Xu 1, 2 , Lei Xin 1, 2 , Jixing Zhao 3 , Shixue Yang 3 , Biao Hong 3 , Yanli Tan 4, 5 , Jie Zhang 5 , Xiang Li 5 , Jie Li 6 , Chunsheng Kang 3 , Chuan Fang 1, 2
Affiliation
|
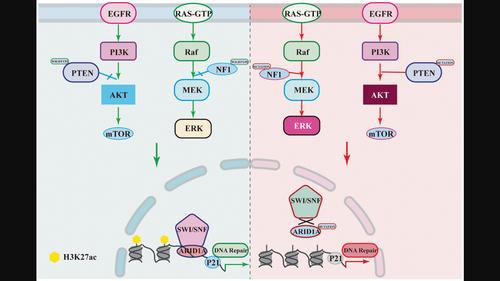
AimsTo investigate the key factors influencing glioma progression and the emergence of treatment resistance by examining the intrinsic connection between mutations in DNA damage and repair‐related genes and the development of chemoresistance in gliomas.MethodsWe conducted a comprehensive analysis of deep‐targeted gene sequencing data from 228 glioma samples. This involved identifying differentially mutated genes across various glioma grades, assessing their functions, and employing I‐TASSER for homology modeling. We elucidated the functional changes induced by high‐frequency site mutations in these genes and investigated their impact on glioma progression.ResultsThe analysis of sequencing mutation results of deep targeted genes in integration revealed that ARID1A gene mutation occurs frequently in glioblastoma and alteration of ARID1A could affect the tolerance of glioma cells to temozolomide treatment. The deletion of proline at position 16 in the ARID1A protein affected the stability of binding of the SWI/SNF core subunit BRG1, which in turn affected the stability of the SWI/SNF complex and led to altered histone modifications in the CDKN1A promoter region, thereby affecting the biological activity of glioma cells, as inferred from modeling and protein interaction analysis.ConclusionThe ARID1A gene is a critical predictive biomarker for glioma. Mutations at the ARID1A locus alter the stability of the SWI/SNF complex, leading to changes in transcriptional regulation in glioma cells. This contributes to an increased malignant phenotype of GBM and plays a pivotal role in mediating chemoresistance.
中文翻译:

深度靶向基因测序揭示 ARID1A 突变是胶质母细胞瘤的重要驱动因素
目的 通过检查DNA损伤和修复相关基因的突变与胶质瘤化疗耐药发生之间的内在联系,探讨影响胶质瘤进展和治疗耐药出现的关键因素。方法我们对来自胶质瘤的深度靶向基因测序数据进行了全面分析。 228 个神经胶质瘤样本。这涉及识别不同神经胶质瘤级别的差异突变基因,评估其功能,并使用 I-TASSER 进行同源建模。我们阐明了这些基因中高频位点突变引起的功能变化,并研究了它们对胶质瘤进展的影响。结果整合中深度靶向基因的测序突变结果分析表明,ARID1A基因突变在胶质母细胞瘤中频繁发生,ARID1A的改变可能会影响胶质瘤的进展。神经胶质瘤细胞对替莫唑胺治疗的耐受性。 ARID1A蛋白16位脯氨酸的缺失影响了SWI/SNF核心亚基BRG1结合的稳定性,进而影响了SWI/SNF复合物的稳定性,并导致CDKN1A启动子区域的组蛋白修饰发生改变,从而根据建模和蛋白质相互作用分析推断,ARID1A 基因会影响神经胶质瘤细胞的生物活性。结论 ARID1A 基因是神经胶质瘤的关键预测生物标志物。 ARID1A 位点的突变改变了 SWI/SNF 复合物的稳定性,导致神经胶质瘤细胞转录调控的变化。这导致 GBM 恶性表型增加,并在介导化疗耐药性中发挥关键作用。
更新日期:2024-04-11
中文翻译:

深度靶向基因测序揭示 ARID1A 突变是胶质母细胞瘤的重要驱动因素
目的 通过检查DNA损伤和修复相关基因的突变与胶质瘤化疗耐药发生之间的内在联系,探讨影响胶质瘤进展和治疗耐药出现的关键因素。方法我们对来自胶质瘤的深度靶向基因测序数据进行了全面分析。 228 个神经胶质瘤样本。这涉及识别不同神经胶质瘤级别的差异突变基因,评估其功能,并使用 I-TASSER 进行同源建模。我们阐明了这些基因中高频位点突变引起的功能变化,并研究了它们对胶质瘤进展的影响。结果整合中深度靶向基因的测序突变结果分析表明,ARID1A基因突变在胶质母细胞瘤中频繁发生,ARID1A的改变可能会影响胶质瘤的进展。神经胶质瘤细胞对替莫唑胺治疗的耐受性。 ARID1A蛋白16位脯氨酸的缺失影响了SWI/SNF核心亚基BRG1结合的稳定性,进而影响了SWI/SNF复合物的稳定性,并导致CDKN1A启动子区域的组蛋白修饰发生改变,从而根据建模和蛋白质相互作用分析推断,ARID1A 基因会影响神经胶质瘤细胞的生物活性。结论 ARID1A 基因是神经胶质瘤的关键预测生物标志物。 ARID1A 位点的突变改变了 SWI/SNF 复合物的稳定性,导致神经胶质瘤细胞转录调控的变化。这导致 GBM 恶性表型增加,并在介导化疗耐药性中发挥关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号