当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Evaluation of Novel Quinazoline Derivatives Possessing a Trifluoromethyl Moiety as Potential Antitumor Agents
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2024-04-11 , DOI: 10.1002/cbdv.202301776 Mingxiu Chen 1, 2 , Sha Cheng 1, 2 , Xing Dai 1, 2, 3 , Jia Yu 1, 2 , HuiDi Wang 4 , BiXue Xu 1, 2 , Heng Luo 1, 2 , Guangcan Xu 1, 2
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2024-04-11 , DOI: 10.1002/cbdv.202301776 Mingxiu Chen 1, 2 , Sha Cheng 1, 2 , Xing Dai 1, 2, 3 , Jia Yu 1, 2 , HuiDi Wang 4 , BiXue Xu 1, 2 , Heng Luo 1, 2 , Guangcan Xu 1, 2
Affiliation
|
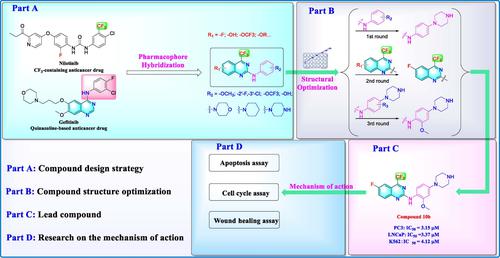
A novel series of trifluoromethyl‐containing quinazoline derivatives with a variety of functional groups was designed, synthesized, and tested for their antitumor activity by following a pharmacophore hybridization strategy. Most of the 20 compounds displayed moderate to excellent antiproliferative activity against five different cell lines (PC3, LNCaP, K562, HeLa, and A549). After three rounds of screening and structural optimization, compound 10 b was identified as the most potent one, with IC50 values of 3.02, 3.45, and 3.98 μM against PC3, LNCaP, and K562 cells, respectively, which were comparable to the effect of the positive control gefitinib. To further explore the mechanism of action of 10 b against cancer, experiments focusing on apoptosis induction, cell cycle arrest, and cell migration assay were conducted. The results showed that 10 b was able to induce apoptosis and prevent tumor cell migration, but had no effect on the cell cycle of tumor cells.
中文翻译:

具有三氟甲基部分的新型喹唑啉衍生物作为潜在抗肿瘤药物的设计、合成和生物学评价
通过遵循药效团杂交策略,设计、合成了一系列具有多种官能团的新型含三氟甲基喹唑啉衍生物,并测试了其抗肿瘤活性。 20 种化合物中的大多数对五种不同的细胞系(PC3、LNCaP、K562、HeLa 和 A549)表现出中等至优异的抗增殖活性。经过三轮筛选和结构优化,化合物 10b 被确定为最有效的化合物,IC 值50 对 PC3、LNCaP 和 K562 细胞的作用值分别为 3.02、3.45 和 3.98 μM,与阳性对照吉非替尼的效果相当。为了进一步探讨 10b 抗癌作用机制,进行了侧重于细胞凋亡诱导、细胞周期阻滞和细胞迁移测定的实验。结果显示10b能够诱导细胞凋亡并阻止肿瘤细胞迁移,但对肿瘤细胞的细胞周期没有影响。
更新日期:2024-04-11
中文翻译:

具有三氟甲基部分的新型喹唑啉衍生物作为潜在抗肿瘤药物的设计、合成和生物学评价
通过遵循药效团杂交策略,设计、合成了一系列具有多种官能团的新型含三氟甲基喹唑啉衍生物,并测试了其抗肿瘤活性。 20 种化合物中的大多数对五种不同的细胞系(PC3、LNCaP、K562、HeLa 和 A549)表现出中等至优异的抗增殖活性。经过三轮筛选和结构优化,化合物 10b 被确定为最有效的化合物,IC 值



























 京公网安备 11010802027423号
京公网安备 11010802027423号