当前位置:
X-MOL 学术
›
Luminescence
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isoquinoline‐based intrinsic fluorescence assessment of erythropoiesis‐stimulating agent, Roxadustat (FG‐4592), in tablets: applications to content uniformity and human plasma evaluation
Luminescence ( IF 2.9 ) Pub Date : 2024-04-12 , DOI: 10.1002/bio.4741 Hany A. Batakoushy 1 , Hani M. Hafez 2 , Marwa M. Soliman 3 , Tahany F. Mohamed 3 , Amal B. Ahmed 4 , Mohamed A. El Hamd 5, 6
Luminescence ( IF 2.9 ) Pub Date : 2024-04-12 , DOI: 10.1002/bio.4741 Hany A. Batakoushy 1 , Hani M. Hafez 2 , Marwa M. Soliman 3 , Tahany F. Mohamed 3 , Amal B. Ahmed 4 , Mohamed A. El Hamd 5, 6
Affiliation
|
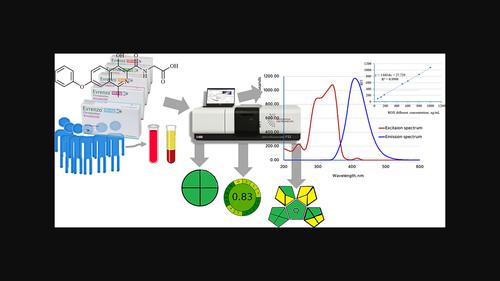
In the present study, a first validated and green spectrofluorimetric approach for its assessment and evaluation in different matrices was investigated. After using an excitation wavelength of 345 nm, Roxadustat (ROX) demonstrates a highly native fluorescence at an emission of 410 nm. The influences of experimental factors such as pH, diluting solvents, and different organized media were tested, and the most appropriate solvent choice was ethanol. It was confirmed that there was a linear relationship between the concentration of ROX and the relative fluorescence intensity in the range 60.0–1000.0 ng ml−1, with the limit of detection and limit of quantitation, respectively, being 17.0 and 53.0 ng ml−1 . The mean recoveries % [±standard deviation (SD), n = 5] for pharmaceutical preparations were 100.11% ± 2.24%, whereas for plasma samples, they were 100.08 ± 1.08% (±SD, n = 5). The results obtained after the application of four greenness criteria, Analytical Eco‐Scale metric, NEMI, GAPI, and AGREE metric, confirmed its eco‐friendliness. In addition, the whiteness meter (RGB12) confirmed its level of sustainability. The International Council for Harmonisation (ICH) criteria were used to verify the developed method through the study in both spiked plasma samples and content uniformity evaluation. An appropriate standard for various applications in industry and quality control laboratories was developed.
中文翻译:

基于异喹啉的片剂中红细胞生成刺激剂 Roxadustat (FG-4592) 的内在荧光评估:在含量均匀性和人血浆评估中的应用
在本研究中,研究了第一个经过验证的绿色荧光分光光度法,用于在不同基质中进行评估和评价。使用 345 nm 的激发波长后,Roxadustat (ROX) 在 410 nm 的发射波长处表现出高度天然的荧光。测试了pH、稀释溶剂和不同组织介质等实验因素的影响,最合适的溶剂选择是乙醇。证实ROX浓度与相对荧光强度在60.0~1000.0 ng ml-1范围内呈线性关系−1, 检测限和定量限分别为17.0和53.0 ng ml−1 。平均回收率 % [±标准差 (SD),n = 5]药物制剂为100.11%±2.24%,而血浆样品为100.08±1.08%(±SD,n = 5)。应用分析生态尺度指标、NEMI、GAPI 和 AGREE 指标这四种绿色标准后获得的结果证实了其生态友好性。此外,白度计(RGB12)证实了其可持续性水平。国际协调委员会 (ICH) 标准用于通过加标血浆样品研究和含量均匀性评估来验证所开发的方法。为工业和质量控制实验室的各种应用制定了适当的标准。
更新日期:2024-04-12
中文翻译:

基于异喹啉的片剂中红细胞生成刺激剂 Roxadustat (FG-4592) 的内在荧光评估:在含量均匀性和人血浆评估中的应用
在本研究中,研究了第一个经过验证的绿色荧光分光光度法,用于在不同基质中进行评估和评价。使用 345 nm 的激发波长后,Roxadustat (ROX) 在 410 nm 的发射波长处表现出高度天然的荧光。测试了pH、稀释溶剂和不同组织介质等实验因素的影响,最合适的溶剂选择是乙醇。证实ROX浓度与相对荧光强度在60.0~1000.0 ng ml-1范围内呈线性关系



























 京公网安备 11010802027423号
京公网安备 11010802027423号