当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Radiosensitizing effect of a novel CTSS inhibitor by enhancing BRCA1 protein stability in triple‐negative breast cancer cells
Cancer Science ( IF 5.7 ) Pub Date : 2024-04-13 , DOI: 10.1111/cas.16174 Eun Choi 1 , Kyung‐Hwa Jeon 1 , Hanhee Lee 1 , Gil‐Im Mun 1 , Jeong‐Ahn Kim 1 , Jae‐Ho Shin 2 , Youngjoo Kwon 1 , Younghwa Na 2 , Yun‐Sil Lee 1
Cancer Science ( IF 5.7 ) Pub Date : 2024-04-13 , DOI: 10.1111/cas.16174 Eun Choi 1 , Kyung‐Hwa Jeon 1 , Hanhee Lee 1 , Gil‐Im Mun 1 , Jeong‐Ahn Kim 1 , Jae‐Ho Shin 2 , Youngjoo Kwon 1 , Younghwa Na 2 , Yun‐Sil Lee 1
Affiliation
|
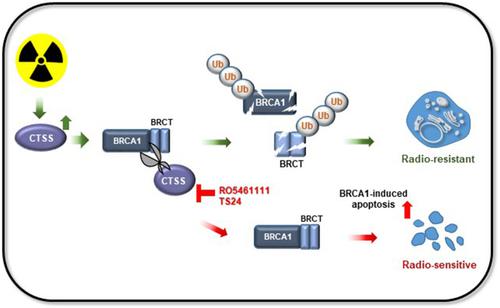
Triple‐negative breast cancer (TNBC) patients harboring wild‐type breast cancer susceptibility gene 1 (BRCA1) account for most TNBC patients but lack adequate targeted therapeutic options. Although radiotherapy (RT) is the primary treatment modality for TNBC patients, radioresistance is one of the major challenges. RT‐induced increase in cathepsin S (CTSS) causes radioresistance through suppressing BRCA1‐mediated apoptosis of tumor cells, which was induced by CTSS‐mediated degradation of BRCA1. Targeting CTSS may provide a novel therapeutic opportunity for TNBC patients. Publicly available data and human tissue microarray slides were analyzed to investigate the relationship between CTSS and BRCA1 in breast cancer patients. A CTSS enzyme assay and in silico docking analysis were conducted to identify a novel CTSS inhibitor. RO5461111 was used first to confirm the concept of targeting CTSS for radiosensitizing effects. The MDA‐MB‐231 TNBC cell line was used for in vitro and in vivo assays. Western blotting, promoter assay, cell death assay, clonogenic survival assay, and immunohistochemistry staining were conducted to evaluate novel CTSS inhibitors. CTSS inhibitors were further evaluated for their additional benefit of inhibiting cell migration. A novel CTSS inhibitor, TS‐24, increased BRCA1 protein levels and showed radiosensitization in TNBC cells with wild‐type BRCA1 and in vivo in a TNBC xenograft mouse model. These effects were attributed by BRCA1‐mediated apoptosis facilitated by TS‐24. Furthermore, TS‐24 demonstrated the additional effect of inhibiting cell migration. Our study suggests that employing CTSS inhibitors for the functional restoration of BRCA1 to enhance RT‐induced apoptosis may provide a novel therapeutic opportunity for TNBC patients harboring wild‐type BRCA1.
中文翻译:

新型 CTSS 抑制剂通过增强三阴性乳腺癌细胞中 BRCA1 蛋白稳定性的放射增敏作用
携带野生型乳腺癌易感基因1(BRCA1)的三阴性乳腺癌(TNBC)患者占大多数TNBC患者,但缺乏足够的靶向治疗选择。尽管放疗 (RT) 是 TNBC 患者的主要治疗方式,但放疗耐药性是主要挑战之一。 RT 诱导的组织蛋白酶 S (CTSS) 增加通过抑制 BRCA1 介导的肿瘤细胞凋亡来引起放射抗性,而肿瘤细胞凋亡是由 CTSS 介导的 BRCA1 降解诱导的。针对 CTSS 可能为 TNBC 患者提供新的治疗机会。对公开数据和人体组织微阵列载玻片进行分析,以研究乳腺癌患者中 CTSS 和 BRCA1 之间的关系。通过 CTSS 酶测定和计算机对接分析来鉴定新型 CTSS 抑制剂。 RO5461111首先用于确认针对CTSS的放射增敏作用的概念。 MDA-MB-231 TNBC 细胞系用于体外和体内测定。通过蛋白质印迹、启动子测定、细胞死亡测定、克隆存活测定和免疫组织化学染色来评估新型 CTSS 抑制剂。 CTSS 抑制剂抑制细胞迁移的额外益处得到了进一步评估。一种新型 CTSS 抑制剂 TS-24 可增加 BRCA1 蛋白水平,并在野生型 BRCA1 的 TNBC 细胞中和 TNBC 异种移植小鼠模型中表现出放射增敏作用。这些效应归因于 TS-24 促进的 BRCA1 介导的细胞凋亡。此外,TS-24 还表现出抑制细胞迁移的额外作用。我们的研究表明,使用 CTSS 抑制剂恢复 BRCA1 的功能以增强 RT 诱导的细胞凋亡可能为携带野生型 BRCA1 的 TNBC 患者提供新的治疗机会。
更新日期:2024-04-13
中文翻译:

新型 CTSS 抑制剂通过增强三阴性乳腺癌细胞中 BRCA1 蛋白稳定性的放射增敏作用
携带野生型乳腺癌易感基因1(BRCA1)的三阴性乳腺癌(TNBC)患者占大多数TNBC患者,但缺乏足够的靶向治疗选择。尽管放疗 (RT) 是 TNBC 患者的主要治疗方式,但放疗耐药性是主要挑战之一。 RT 诱导的组织蛋白酶 S (CTSS) 增加通过抑制 BRCA1 介导的肿瘤细胞凋亡来引起放射抗性,而肿瘤细胞凋亡是由 CTSS 介导的 BRCA1 降解诱导的。针对 CTSS 可能为 TNBC 患者提供新的治疗机会。对公开数据和人体组织微阵列载玻片进行分析,以研究乳腺癌患者中 CTSS 和 BRCA1 之间的关系。通过 CTSS 酶测定和计算机对接分析来鉴定新型 CTSS 抑制剂。 RO5461111首先用于确认针对CTSS的放射增敏作用的概念。 MDA-MB-231 TNBC 细胞系用于体外和体内测定。通过蛋白质印迹、启动子测定、细胞死亡测定、克隆存活测定和免疫组织化学染色来评估新型 CTSS 抑制剂。 CTSS 抑制剂抑制细胞迁移的额外益处得到了进一步评估。一种新型 CTSS 抑制剂 TS-24 可增加 BRCA1 蛋白水平,并在野生型 BRCA1 的 TNBC 细胞中和 TNBC 异种移植小鼠模型中表现出放射增敏作用。这些效应归因于 TS-24 促进的 BRCA1 介导的细胞凋亡。此外,TS-24 还表现出抑制细胞迁移的额外作用。我们的研究表明,使用 CTSS 抑制剂恢复 BRCA1 的功能以增强 RT 诱导的细胞凋亡可能为携带野生型 BRCA1 的 TNBC 患者提供新的治疗机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号