当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into Li+ adsorption using H2TiO3 in salt lakes with different hydrochemical types: The activity of OH groups
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2024-04-04 , DOI: 10.1016/j.jiec.2024.04.007 Qiao Liu , Yuxuan Du , Meng Liu , Xiaoping Li , Zonghan Huang , Songjun Guo , Rongzhi Chen
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2024-04-04 , DOI: 10.1016/j.jiec.2024.04.007 Qiao Liu , Yuxuan Du , Meng Liu , Xiaoping Li , Zonghan Huang , Songjun Guo , Rongzhi Chen
|
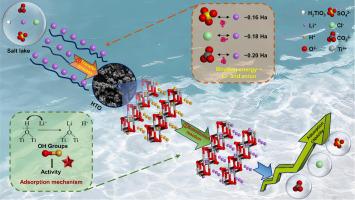
Adsorption-based lithium (Li) recovery from salt lakes has focused on Li selectivity in competitive cations and improving the adsorption capacity, but ignored the effect of different hydrochemical types on its adsorption. Herein, the adsorption behavior of Li was comparatively studied in carbonate-type, sulfate-type, and chloride-type salt lakes using HTiO as an adsorbent. The Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy confirmed that the replacement of O–H by O-Li bond in HTiO sieves drove the Li capture. At a salt concentration of 10 g/L, the adsorption of Li by HTiO was in the order of SO (40.08 mg g) > Cl (36.66 mg g) > CO (30.18 mg g). Thermogravimetric analysis (TGA) and X-ray photoelectron spectroscopy (XPS) demonstrated that the number of activated OH groups in the three salt solutions followed the same order of adsorption capacity. The binding energy of Li and anion calculated by density functional theory (DFT) verified the activity of OH groups, further revealing the influence mechanism of hydrochemical types on Li adsorption. This study suggests that the activity of Li adsorption sites was affected by the specific hydrochemical types of salt lakes, providing theoretical guidance for the Li adsorptive recovery from different water matrices.
中文翻译:

深入了解不同水化学类型盐湖中 H2TiO3 对 Li+ 的吸附:OH 基团的活性
基于吸附的盐湖锂(Li)回收主要关注锂在竞争性阳离子中的选择性和提高吸附容量,但忽略了不同水化学类型对其吸附的影响。本文以HTiO为吸附剂,比较研究了Li在碳酸盐型、硫酸盐型和氯化物型盐湖中的吸附行为。傅里叶变换红外光谱(FTIR)和拉曼光谱证实,HTiO 筛中 O-Li 键取代 O-H 驱动了 Li 的捕获。在盐浓度为10 g/L时,HTiO对Li的吸附顺序为SO(40.08 mg g-1)> Cl(36.66 mg g-1)> CO(30.18 mg g-1)。热重分析(TGA)和X射线光电子能谱(XPS)表明三种盐溶液中活化OH基团的数量遵循相同的吸附容量顺序。通过密度泛函理论(DFT)计算的Li与阴离子的结合能验证了OH基团的活性,进一步揭示了水化学类型对Li吸附的影响机制。该研究表明,锂吸附位点的活性受到盐湖特定水化学类型的影响,为不同水基质中锂吸附回收提供理论指导。
更新日期:2024-04-04
中文翻译:

深入了解不同水化学类型盐湖中 H2TiO3 对 Li+ 的吸附:OH 基团的活性
基于吸附的盐湖锂(Li)回收主要关注锂在竞争性阳离子中的选择性和提高吸附容量,但忽略了不同水化学类型对其吸附的影响。本文以HTiO为吸附剂,比较研究了Li在碳酸盐型、硫酸盐型和氯化物型盐湖中的吸附行为。傅里叶变换红外光谱(FTIR)和拉曼光谱证实,HTiO 筛中 O-Li 键取代 O-H 驱动了 Li 的捕获。在盐浓度为10 g/L时,HTiO对Li的吸附顺序为SO(40.08 mg g-1)> Cl(36.66 mg g-1)> CO(30.18 mg g-1)。热重分析(TGA)和X射线光电子能谱(XPS)表明三种盐溶液中活化OH基团的数量遵循相同的吸附容量顺序。通过密度泛函理论(DFT)计算的Li与阴离子的结合能验证了OH基团的活性,进一步揭示了水化学类型对Li吸附的影响机制。该研究表明,锂吸附位点的活性受到盐湖特定水化学类型的影响,为不同水基质中锂吸附回收提供理论指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号