当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
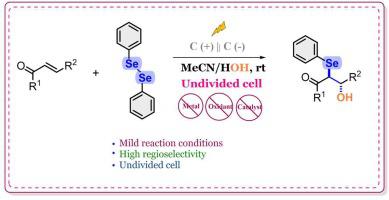
Electrochemically driven regioselective organoselenation for selective synthesis of β- hydroxy substituted selanylated ketones
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.tetlet.2024.155051 Musarrat Fatma , Faiz Ahmed Khan
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.tetlet.2024.155051 Musarrat Fatma , Faiz Ahmed Khan
|
A simple yet efficient approach towards the synthesis of -hydroxy selanylated ketones was developed from easily accessible chalcones and diphenyl diselenide through an electrochemical pathway. In this technique, diphenyl diselenide and water are the source of PhSe and hydroxyl (–OH) groups that can be inserted at the and positions respectively producing -hydroxy selanylated ketones in moderate to good yields. The reaction features difunctionalization of ketones, catalyst free, mild reaction conditions, stereo and regioselectivity in an undivided electrochemical cell at room temperature. For this transformation, a plausible radical mechanism has been put out.
中文翻译:

电化学驱动的区域选择性有机硒化用于选择性合成β-羟基取代的硒酰化酮
通过电化学途径,从容易获得的查尔酮和二苯基二硒化物中开发出一种简单而有效的合成β-羟基硒酰化酮的方法。在该技术中,二苯基二硒化物和水是PhSe和羟基(-OH)基团的来源,它们可以分别插入到 和 位置,以中等至良好的产率产生-羟基硒酰化酮。该反应具有酮双官能化、无催化剂、反应条件温和、室温下在完整电化学电池中具有立体和区域选择性的特点。对于这种转变,已经提出了一个看似合理的激进机制。
更新日期:2024-04-06
中文翻译:

电化学驱动的区域选择性有机硒化用于选择性合成β-羟基取代的硒酰化酮
通过电化学途径,从容易获得的查尔酮和二苯基二硒化物中开发出一种简单而有效的合成β-羟基硒酰化酮的方法。在该技术中,二苯基二硒化物和水是PhSe和羟基(-OH)基团的来源,它们可以分别插入到 和 位置,以中等至良好的产率产生-羟基硒酰化酮。该反应具有酮双官能化、无催化剂、反应条件温和、室温下在完整电化学电池中具有立体和区域选择性的特点。对于这种转变,已经提出了一个看似合理的激进机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号