当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Cu doping on Ni surface on CO formation pathways during the methane dry reforming reaction
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-04-09 , DOI: 10.1016/j.mcat.2024.114125 Cunxin Zhang , Juntian Niu , Baihe Guo , Haiyu Liu , Yan Jin , Jingyu Ran
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-04-09 , DOI: 10.1016/j.mcat.2024.114125 Cunxin Zhang , Juntian Niu , Baihe Guo , Haiyu Liu , Yan Jin , Jingyu Ran
|
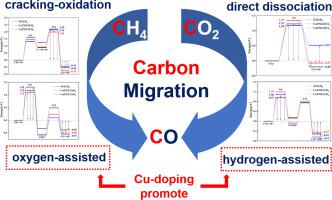
Three different Cu-Ni/ZrO surfaces are constructed in this paper to investigate the variation rules of carbon migration paths when CH and CO act as the carbon source. Actually, Cu doping can affect the migration pathway of carbon atom from CH and CO to CO. When CH is the carbon source, the carbon migration process is mainly accomplished through the oxygen-assisted pathway, and Cu doping shows no effect on the dominance of the oxygen-assisted pathway. However, the carbon migration process mainly follows the hydrogen-assisted pathway when CO is the carbon source. Meanwhile, the activation energy gap between the hydrogen-assisted pathway and the direct dissociation pathway is reduced by Cu doping. The reduction of activation energy gap proves that the mode of carbon migration of CO from the dominance of a single pathway to the competition of a dual pathway. Furthermore, the active energy of oxidation of CH species is always lower compared with deep cracking. The difference of the activation energy of two reaction process of CH species raises up as Cu doping ratio increases. The expansion of difference of activation energy proves that Cu doping can induce CH species to prefer to be oxidized rather than direct cracking, lowering the generation of deposited carbon atoms. In addition, Cu doping promotes the oxidation process of surface carbon atoms and improves the anti-carbon deposition ability of the catalyst surface. Moreover, the order of adsorption strength of CO on different Cu-Ni/ZrO is: Ni/ZrO> Cu1Ni2/ZrO> Cu2Ni1/ZrO. And the charge transfer from the catalyst surface to CO molecules is also reduced by Cu doping. Therefore, the interaction between CO and the catalyst surface can be weakened by Cu doping, which is conducive to the desorption of CO molecule.
中文翻译:

Ni表面Cu掺杂对甲烷干重整反应过程中CO生成途径的影响
本文构建了三种不同的Cu-Ni/ZrO表面,研究CH和CO作为碳源时碳迁移路径的变化规律。实际上,Cu掺杂可以影响碳原子从CH和CO到CO的迁移途径。当CH为碳源时,碳迁移过程主要通过氧辅助途径完成,而Cu掺杂对碳原子的主导地位没有影响。氧气辅助途径。然而,当CO为碳源时,碳迁移过程主要遵循氢辅助途径。同时,Cu掺杂减小了氢辅助途径和直接解离途径之间的活化能差距。活化能差的减小证明CO的碳迁移模式从单一途径的主导转向双途径的竞争。此外,与深度裂解相比,CH物质的氧化活性能始终较低。随着Cu掺杂比例的增加,CH物种两个反应过程的活化能差异增大。活化能差异的扩大证明Cu掺杂可以诱导CH物种更倾向于被氧化而不是直接裂解,从而减少沉积碳原子的生成。此外,Cu掺杂促进了表面碳原子的氧化过程,提高了催化剂表面的抗积碳能力。此外,不同Cu-Ni/ZrO对CO的吸附强度顺序为:Ni/ZrO>Cu1Ni2/ZrO>Cu2Ni1/ZrO。 Cu掺杂也减少了从催化剂表面到CO分子的电荷转移。因此,Cu掺杂可以减弱CO与催化剂表面的相互作用,有利于CO分子的解吸。
更新日期:2024-04-09
中文翻译:

Ni表面Cu掺杂对甲烷干重整反应过程中CO生成途径的影响
本文构建了三种不同的Cu-Ni/ZrO表面,研究CH和CO作为碳源时碳迁移路径的变化规律。实际上,Cu掺杂可以影响碳原子从CH和CO到CO的迁移途径。当CH为碳源时,碳迁移过程主要通过氧辅助途径完成,而Cu掺杂对碳原子的主导地位没有影响。氧气辅助途径。然而,当CO为碳源时,碳迁移过程主要遵循氢辅助途径。同时,Cu掺杂减小了氢辅助途径和直接解离途径之间的活化能差距。活化能差的减小证明CO的碳迁移模式从单一途径的主导转向双途径的竞争。此外,与深度裂解相比,CH物质的氧化活性能始终较低。随着Cu掺杂比例的增加,CH物种两个反应过程的活化能差异增大。活化能差异的扩大证明Cu掺杂可以诱导CH物种更倾向于被氧化而不是直接裂解,从而减少沉积碳原子的生成。此外,Cu掺杂促进了表面碳原子的氧化过程,提高了催化剂表面的抗积碳能力。此外,不同Cu-Ni/ZrO对CO的吸附强度顺序为:Ni/ZrO>Cu1Ni2/ZrO>Cu2Ni1/ZrO。 Cu掺杂也减少了从催化剂表面到CO分子的电荷转移。因此,Cu掺杂可以减弱CO与催化剂表面的相互作用,有利于CO分子的解吸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号