当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
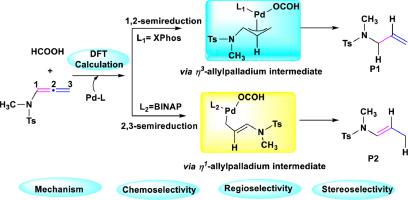
Interplay of Ligand-Controlled Reaction Pathways: DFT Analysis of Mechanisms and Selectivities in Pd-Catalyzed Semireduction of Allenamides
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-04-12 , DOI: 10.1016/j.mcat.2024.114126 Ji Ma , Simeng Qi , Guowei Yan , Alexander M. Kirillov , Lizi Yang , Ran Fang
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-04-12 , DOI: 10.1016/j.mcat.2024.114126 Ji Ma , Simeng Qi , Guowei Yan , Alexander M. Kirillov , Lizi Yang , Ran Fang
|
The ligand-controlled mechanisms and chemo-, regio- and stereoselectivities were theoretically investigated in the Pd-catalyzed semireduction of allenamides. Two different ligands, including monodentate XPhos and bidentate BINAP, were selected as model systems in the DFT calculations. For both types of ligand systems, the obtained results clearly indicate that the first step of the reaction involves the formation of a Pd-hydride intermediate via the oxidative addition of formic acid to Pd(II) precursor. For a monodentate XPhos ligand, the hydride insertion between a Pd-hydride intermediate and allenamide generates an -allyl palladium intermediate and gives a 1,2-semireduction product through the -H and reductive eliminations. In the reaction with BINAP, an -allyl palladium intermediate is converted into a more stable -allyl palladium intermediate, leading to the 2,3-semireduction product via the -hydride elimination. The obtained theoretical evidence suggests that an -- interconvertible intermediate plays a vital role in determining the selectivity. Furthermore, the analysis of independent gradient model based on Hirshfeld partition (IGMH) as well as atoms-in-molecules (AIM) analysis indicate that the regioselectivity and stereoselectivity are influenced by different CH···π and π···π stacking interactions. The present work contributes to the understanding of the mechanisms and may assist in the design of prospective catalysts to control the regio- and stereoselectivities in this type of important organic transformations.
中文翻译:

配体控制反应途径的相互作用:Pd 催化烯丙酰胺半还原反应机理和选择性的 DFT 分析
在 Pd 催化的联二烯酰胺半还原反应中,从理论上研究了配体控制机制以及化学、区域和立体选择性。选择两种不同的配体,包括单齿 XPhos 和双齿 BINAP,作为 DFT 计算中的模型系统。对于两种类型的配体系统,获得的结果清楚地表明反应的第一步涉及通过甲酸与 Pd(II) 前体的氧化加成形成 Pd-氢化物中间体。对于单齿 XPhos 配体,Pd 氢化物中间体和丙二烯酰胺之间的氢化物插入生成 β-烯丙基钯中间体,并通过 -H 和还原消除得到 1,2-半还原产物。在与 BINAP 的反应中,β-烯丙基钯中间体转化为更稳定的β-烯丙基钯中间体,通过β-氢化物消除产生 2,3-半还原产物。获得的理论证据表明,可相互转化的中间体在决定选择性方面起着至关重要的作用。此外,基于赫什菲尔德分配(IGMH)的独立梯度模型分析以及分子中原子(AIM)分析表明,区域选择性和立体选择性受到不同CH·π和π·π堆积相互作用的影响。目前的工作有助于理解其机制,并可能有助于设计潜在的催化剂来控制此类重要有机转化中的区域选择性和立体选择性。
更新日期:2024-04-12
中文翻译:

配体控制反应途径的相互作用:Pd 催化烯丙酰胺半还原反应机理和选择性的 DFT 分析
在 Pd 催化的联二烯酰胺半还原反应中,从理论上研究了配体控制机制以及化学、区域和立体选择性。选择两种不同的配体,包括单齿 XPhos 和双齿 BINAP,作为 DFT 计算中的模型系统。对于两种类型的配体系统,获得的结果清楚地表明反应的第一步涉及通过甲酸与 Pd(II) 前体的氧化加成形成 Pd-氢化物中间体。对于单齿 XPhos 配体,Pd 氢化物中间体和丙二烯酰胺之间的氢化物插入生成 β-烯丙基钯中间体,并通过 -H 和还原消除得到 1,2-半还原产物。在与 BINAP 的反应中,β-烯丙基钯中间体转化为更稳定的β-烯丙基钯中间体,通过β-氢化物消除产生 2,3-半还原产物。获得的理论证据表明,可相互转化的中间体在决定选择性方面起着至关重要的作用。此外,基于赫什菲尔德分配(IGMH)的独立梯度模型分析以及分子中原子(AIM)分析表明,区域选择性和立体选择性受到不同CH·π和π·π堆积相互作用的影响。目前的工作有助于理解其机制,并可能有助于设计潜在的催化剂来控制此类重要有机转化中的区域选择性和立体选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号