当前位置:
X-MOL 学术
›
J. Mol. Cell. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deficiency of smooth muscle cell ILF3 alleviates intimal hyperplasia via HMGB1 mRNA degradation-mediated regulation of the STAT3/DUSP16 axis
Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.yjmcc.2024.04.004 Ya-min Hou , Bo-han Xu , Qiu-ting Zhang , Jie Cheng , Xu Zhang , Hong-rui Yang , Ze-ying Wang , Peng Wang , Ming-xiang Zhang
Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.yjmcc.2024.04.004 Ya-min Hou , Bo-han Xu , Qiu-ting Zhang , Jie Cheng , Xu Zhang , Hong-rui Yang , Ze-ying Wang , Peng Wang , Ming-xiang Zhang
|
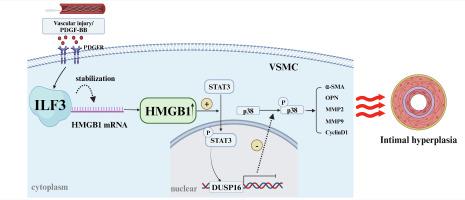
Intimal hyperplasia is a complicated pathophysiological phenomenon attributable to in-stent restenosis, and the underlying mechanism remains unclear. Interleukin enhancer-binding factor 3 (ILF3), a double-stranded RNA-binding protein involved in regulating mRNA stability, has been recently demonstrated to assume a crucial role in cardiovascular disease; nevertheless, its impact on intimal hyperplasia remains unknown. In current study, we used samples of human restenotic arteries and rodent models of intimal hyperplasia, we found that vascular smooth muscle cell (VSMC) ILF3 expression was markedly elevated in human restenotic arteries and murine ligated carotid arteries. SMC-specific ILF3 knockout mice significantly suppressed injury induced neointimal formation. In vitro, platelet-derived growth factor type BB (PDGF-BB) treatment elevated the level of VSMC ILF3 in a dose- and time-dependent manner. ILF3 silencing markedly inhibited PDGF-BB-induced phenotype switching, proliferation, and migration in VSMCs. Transcriptome sequencing and RNA immunoprecipitation sequencing depicted that ILF3 maintained its stability upon binding to the mRNA of the high-mobility group box 1 protein (HMGB1), thereby exerting an inhibitory effect on the transcription of dual specificity phosphatase 16 (DUSP16) through enhanced phosphorylation of signal transducer and activator of transcription 3 (STAT3). Therefore, the results both in vitro and in vivo indicated that the loss of ILF3 in VSMC ameliorated neointimal hyperplasia by regulating the STAT3/DUSP16 axis through the degradation of HMGB1 mRNA. Our findings revealed that vascular injury activates VSMC ILF3, which in turn promotes intima formation. Consequently, targeting specific VSMC ILF3 may present a potential therapeutic strategy for ameliorating cardiovascular restenosis.
中文翻译:

平滑肌细胞 ILF3 缺乏通过 HMGB1 mRNA 降解介导的 STAT3/DUSP16 轴调节减轻内膜增生
内膜增生是支架内再狭窄的一种复杂的病理生理现象,其潜在机制尚不清楚。白细胞介素增强子结合因子 3 (ILF3) 是一种参与调节 mRNA 稳定性的双链 RNA 结合蛋白,最近被证明在心血管疾病中发挥着至关重要的作用。然而,它对内膜增生的影响仍然未知。在本研究中,我们使用人类再狭窄动脉和啮齿动物内膜增生模型样本,发现血管平滑肌细胞(VSMC)ILF3在人类再狭窄动脉和小鼠结扎颈动脉中表达显着升高。 SMC特异性ILF3敲除小鼠显着抑制损伤诱导的新内膜形成。在体外,血小板衍生生长因子 BB 型 (PDGF-BB) 治疗以剂量和时间依赖性方式升高 VSMC ILF3 水平。 ILF3 沉默显着抑制 PDGF-BB 诱导的 VSMC 中表型转换、增殖和迁移。转录组测序和RNA免疫沉淀测序表明,ILF3在与高迁移率group box 1蛋白(HMGB1)的mRNA结合后保持稳定性,从而通过增强双特异性磷酸酶16(DUSP16)的磷酸化对双特异性磷酸酶16(DUSP16)的转录产生抑制作用。信号转导子和转录激活子 3 (STAT3)。因此,体外和体内结果表明,VSMC 中 ILF3 的缺失通过降解 HMGB1 mRNA 来调节 STAT3/DUSP16 轴,从而改善新生内膜增生。我们的研究结果表明,血管损伤会激活 VSMC ILF3,进而促进内膜形成。因此,针对特定的 VSMC ILF3 可能为改善心血管再狭窄提供潜在的治疗策略。
更新日期:2024-04-06
中文翻译:

平滑肌细胞 ILF3 缺乏通过 HMGB1 mRNA 降解介导的 STAT3/DUSP16 轴调节减轻内膜增生
内膜增生是支架内再狭窄的一种复杂的病理生理现象,其潜在机制尚不清楚。白细胞介素增强子结合因子 3 (ILF3) 是一种参与调节 mRNA 稳定性的双链 RNA 结合蛋白,最近被证明在心血管疾病中发挥着至关重要的作用。然而,它对内膜增生的影响仍然未知。在本研究中,我们使用人类再狭窄动脉和啮齿动物内膜增生模型样本,发现血管平滑肌细胞(VSMC)ILF3在人类再狭窄动脉和小鼠结扎颈动脉中表达显着升高。 SMC特异性ILF3敲除小鼠显着抑制损伤诱导的新内膜形成。在体外,血小板衍生生长因子 BB 型 (PDGF-BB) 治疗以剂量和时间依赖性方式升高 VSMC ILF3 水平。 ILF3 沉默显着抑制 PDGF-BB 诱导的 VSMC 中表型转换、增殖和迁移。转录组测序和RNA免疫沉淀测序表明,ILF3在与高迁移率group box 1蛋白(HMGB1)的mRNA结合后保持稳定性,从而通过增强双特异性磷酸酶16(DUSP16)的磷酸化对双特异性磷酸酶16(DUSP16)的转录产生抑制作用。信号转导子和转录激活子 3 (STAT3)。因此,体外和体内结果表明,VSMC 中 ILF3 的缺失通过降解 HMGB1 mRNA 来调节 STAT3/DUSP16 轴,从而改善新生内膜增生。我们的研究结果表明,血管损伤会激活 VSMC ILF3,进而促进内膜形成。因此,针对特定的 VSMC ILF3 可能为改善心血管再狭窄提供潜在的治疗策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号