当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tethered Helical Ladder-Type Aromatic Lactams
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-16 , DOI: 10.1021/jacs.4c01347 Huidong Xie 1, 2 , Zuo Xiao 1, 2 , Yixiao Song 1, 2 , Ke Jin 1, 2 , Hongxing Liu 1 , Erjun Zhou 1, 2 , Jing Cao 3 , Jiangzhao Chen 4 , Junqiao Ding 5 , Chenyi Yi 6 , Xingxing Shen 7 , Chuantian Zuo 1, 2 , Liming Ding 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-16 , DOI: 10.1021/jacs.4c01347 Huidong Xie 1, 2 , Zuo Xiao 1, 2 , Yixiao Song 1, 2 , Ke Jin 1, 2 , Hongxing Liu 1 , Erjun Zhou 1, 2 , Jing Cao 3 , Jiangzhao Chen 4 , Junqiao Ding 5 , Chenyi Yi 6 , Xingxing Shen 7 , Chuantian Zuo 1, 2 , Liming Ding 1, 2
Affiliation
|
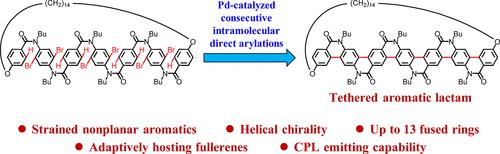
Tethered nonplanar aromatics (TNAs) make up an important class of nonplanar aromatic compounds showing unique features. However, the knowledge on the synthesis, structures, and properties of TNAs remains insufficient. In this work, a new type of TNAs, the tethered aromatic lactams, is synthesized via Pd-catalyzed consecutive intramolecular direct arylations. These molecules possess a helical ladder-type conjugated system of up to 13 fused rings. The overall yields ranged from 3.4 to 4.3%. The largest of the tethered aromatic lactams, 6L-Bu-C14, demonstrates a guest-adaptive hosting capability of TNAs for the first time. When binding fullerene guests, the cavity of 6L-Bu-C14 became more circular to better accommodate spherical fullerene molecules. The host–guest interaction is thoroughly studied by X-ray crystallography, theoretical calculations, fluorescence titration, and nuclear magnetic resonance (NMR) titration experiments. 6L-Bu-C14 shows stronger binding with C70 than with C60 due to the better convex–concave π–π interaction. P and M enantiomers of all tethered aromatic lactams show distinct and persistent chiroptical properties and demonstrate the potential of chiral TNAs as circularly polarized luminescence (CPL) emitters.
中文翻译:

系链螺旋梯型芳香内酰胺
系链非平面芳族化合物 (TNA) 是一类重要的非平面芳族化合物,具有独特的特征。然而,关于 TNA 的合成、结构和性质的知识仍然不足。在这项工作中,一种新型的 TNA,即束缚芳香内酰胺,是通过 Pd 催化的连续分子内直接芳基化合成的。这些分子拥有多达 13 个稠合环的螺旋梯型共轭系统。总收益率在3.4%至4.3%之间。最大的束缚芳香内酰胺6L-Bu-C 14首次展示了 TNA 的客体自适应承载能力。当结合富勒烯客体时, 6L-Bu-C 14的空腔变得更加圆形,以更好地容纳球形富勒烯分子。通过X射线晶体学、理论计算、荧光滴定和核磁共振(NMR)滴定实验对主客体相互作用进行了深入研究。由于更好的凸凹π-π相互作用,6L-Bu-C 14与C 70 的结合比与C 60 的结合更强。所有束缚芳香内酰胺的P和M对映体均表现出独特且持久的手性光学特性,并证明了手性 TNA 作为圆偏振发光 (CPL) 发射体的潜力。
更新日期:2024-04-16
中文翻译:

系链螺旋梯型芳香内酰胺
系链非平面芳族化合物 (TNA) 是一类重要的非平面芳族化合物,具有独特的特征。然而,关于 TNA 的合成、结构和性质的知识仍然不足。在这项工作中,一种新型的 TNA,即束缚芳香内酰胺,是通过 Pd 催化的连续分子内直接芳基化合成的。这些分子拥有多达 13 个稠合环的螺旋梯型共轭系统。总收益率在3.4%至4.3%之间。最大的束缚芳香内酰胺6L-Bu-C 14首次展示了 TNA 的客体自适应承载能力。当结合富勒烯客体时, 6L-Bu-C 14的空腔变得更加圆形,以更好地容纳球形富勒烯分子。通过X射线晶体学、理论计算、荧光滴定和核磁共振(NMR)滴定实验对主客体相互作用进行了深入研究。由于更好的凸凹π-π相互作用,6L-Bu-C 14与C 70 的结合比与C 60 的结合更强。所有束缚芳香内酰胺的P和M对映体均表现出独特且持久的手性光学特性,并证明了手性 TNA 作为圆偏振发光 (CPL) 发射体的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号