Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Native mass spectrometry of complexes formed by molecular glues reveals stoichiometric rearrangement of E3 ligases
Analyst ( IF 4.2 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4an00110a Cara Jackson 1 , Rebecca Beveridge 1
Analyst ( IF 4.2 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4an00110a Cara Jackson 1 , Rebecca Beveridge 1
Affiliation
|
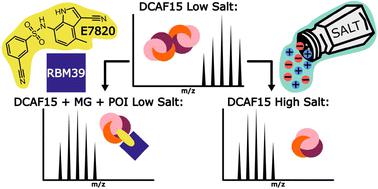
In this application of native mass spectrometry (nMS) to investigate complexes formed by molecular glues (MGs), we have demonstrated its efficiency in delineating stoichiometric rearrangements of E3 ligases that occur during targeted protein degradation (TPD). MGs stabilise interactions between an E3 ligase and a protein of interest (POI) targeted for degradation, and these ternary interactions are challenging to characterise. We have shown that nMS can unambiguously identify complexes formed between the CRBN : DDB1 E3 ligase and the POI GSPT1 upon the addition of lenalidomide, pomalidomide or thalidomide. Ternary complex formation was also identified involving the DCAF15 : DDA1 : DDB1 E3 ligase in the presence of MG (E7820 or indisulam) and POI RBM39. Moreover, we uncovered that the DCAF15 : DDA1 : DDB1 E3 ligase self-associates into dimers and trimers when analysed alone at low salt concentrations (100 mM ammonium acetate) which dissociate into single copies of the complex at higher salt concentrations (500 mM ammonium acetate), or upon the addition of MG and POI, forming a 1 : 1 : 1 ternary complex. This work demonstrates the strength of nMS in TPD research, reveals novel binding mechanisms of the DCAF15 E3 ligase, and its self-association into dimers and trimers at reduced salt concentration during structural analysis.
中文翻译:

分子胶形成的复合物的天然质谱揭示了 E3 连接酶的化学计量重排
在应用天然质谱 (nMS) 来研究分子胶 (MG) 形成的复合物中,我们证明了其在描绘目标蛋白降解 (TPD) 过程中发生的 E3 连接酶化学计量重排方面的效率。 MG 可稳定 E3 连接酶和目标降解蛋白 (POI) 之间的相互作用,而这些三元相互作用很难表征。我们已经证明,在添加来那度胺、泊马度胺或沙利度胺后,nMS 可以明确识别 CRBN : DDB1 E3 连接酶和 POI GSPT1 之间形成的复合物。在 MG(E7820 或 indisulam)和 POI RBM39 存在的情况下,还鉴定了涉及 DCAF15:DDA1:DDB1 E3 连接酶的三元复合物形成。此外,我们发现,当在低盐浓度(100 mM 醋酸铵)下单独分析时,DCAF15 : DDA1 : DDB1 E3 连接酶自缔合成二聚体和三聚体,在较高盐浓度(500 mM 醋酸铵)下解离成复合物的单个拷贝),或者添加MG和POI后,形成1:1:1三元复合物。这项工作展示了 nMS 在 TPD 研究中的优势,揭示了 DCAF15 E3 连接酶的新颖结合机制,及其在结构分析过程中在降低盐浓度的情况下自缔合成二聚体和三聚体。
更新日期:2024-04-19
中文翻译:

分子胶形成的复合物的天然质谱揭示了 E3 连接酶的化学计量重排
在应用天然质谱 (nMS) 来研究分子胶 (MG) 形成的复合物中,我们证明了其在描绘目标蛋白降解 (TPD) 过程中发生的 E3 连接酶化学计量重排方面的效率。 MG 可稳定 E3 连接酶和目标降解蛋白 (POI) 之间的相互作用,而这些三元相互作用很难表征。我们已经证明,在添加来那度胺、泊马度胺或沙利度胺后,nMS 可以明确识别 CRBN : DDB1 E3 连接酶和 POI GSPT1 之间形成的复合物。在 MG(E7820 或 indisulam)和 POI RBM39 存在的情况下,还鉴定了涉及 DCAF15:DDA1:DDB1 E3 连接酶的三元复合物形成。此外,我们发现,当在低盐浓度(100 mM 醋酸铵)下单独分析时,DCAF15 : DDA1 : DDB1 E3 连接酶自缔合成二聚体和三聚体,在较高盐浓度(500 mM 醋酸铵)下解离成复合物的单个拷贝),或者添加MG和POI后,形成1:1:1三元复合物。这项工作展示了 nMS 在 TPD 研究中的优势,揭示了 DCAF15 E3 连接酶的新颖结合机制,及其在结构分析过程中在降低盐浓度的情况下自缔合成二聚体和三聚体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号