European Journal of Clinical Microbiology & Infectious Diseases ( IF 4.5 ) Pub Date : 2024-04-16 , DOI: 10.1007/s10096-024-04830-x Lijuan Wan , Xueqin Cai , Meng Ling , Jinsong Kan , Meiling Yin , Huiyan Wang
|
Purpose
Cancer patients are at heightened risk for invasive aspergillosis (IA), a condition associated with elevated mortality risk. The JF5-based Aspergillus Galactomannoprotein Lateral Flow Device (AspLFD) offers rapid point-of-care testing (POCT) for IA. This study evaluated the diagnostic performance of AspLFD in cancer populations.
Methods
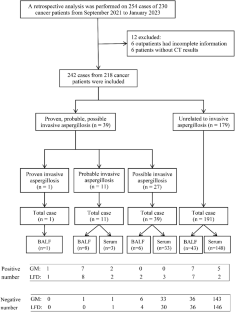
This retrospective study examined cancer patient bronchoalveolar lavage fluid (BALF) and serum samples collected between September 2021 and January 2023. Both AspLFD and galactomannan (GM) assays were conducted, and the results were analysed by two independent researchers.
Results
This study included 242 samples from 218 cancer patients, with 58 BALF and 184 serum samples. The overall agreement between AspLFD and GM assay results was 92.1%, with a kappa value of 0.552. AspLFD diagnosed proven/probable IA with a sensitivity and specificity of 91.7% and 95.3%, respectively, whereas GM exhibited sensitivity and specificity values of 83.3% and 93.7%, respectively. There were no statistical differences in the sensitivity and specificity between the two methods (P > 0.05). For serum analyses, AspLFD and GM exhibited similar sensitivity (66.7% vs. 66.7%, P > 0.05) and specificity (98.6% vs. 96.6%, P > 0.05) values. However, the sensitivity of the AspLFD was superior to the GM assay (100% vs. 88.9%) in BALF analyses but the difference was not statistically significant (P > 0.05), with no difference in specificity (83.7% vs. 83.7%, P > 0.05). In the solid-tumour cohort, both the AspLFD and GM assay exhibited high sensitivity (100% for both) and specificity (94.2% vs. 92.8%, P > 0.05).
Conclusion
The AspLFD demonstrated good performance in diagnosing IA in cancer patients, especially those with solid tumours. The AspLFD is thus an alternative POCT, particularly when GM evaluations are not readily available.
中文翻译:

基于 JF5 的曲霉半乳甘露糖蛋白侧流装置用于诊断癌症患者侵袭性曲霉病的评估
目的
癌症患者患侵袭性曲霉病 (IA) 的风险较高,这种疾病与死亡风险升高相关。基于 JF5 的曲霉半乳甘露糖蛋白侧流装置 (AspLFD) 可为 IA 提供快速即时护理测试 (POCT)。本研究评估了 AspLFD 在癌症人群中的诊断性能。
方法
这项回顾性研究检查了 2021 年 9 月至 2023 年 1 月期间收集的癌症患者支气管肺泡灌洗液 (BALF) 和血清样本。进行了 AspLFD 和半乳甘露聚糖 (GM) 检测,结果由两名独立研究人员进行分析。
结果
这项研究包括来自 218 名癌症患者的 242 个样本,其中包括 58 个 BALF 样本和 184 个血清样本。 AspLFD 和 GM 检测结果的总体一致性为 92.1%,kappa 值为 0.552。 AspLFD 诊断已证实/可能的 IA 的敏感性和特异性分别为 91.7% 和 95.3%,而 GM 的敏感性和特异性值分别为 83.3% 和 93.7%。两种方法的敏感性和特异性均无统计学差异(P >0.05)。对于血清分析,AspLFD 和 GM 表现出相似的敏感性(66.7% vs. 66.7%,P > 0.05)和特异性(98.6% vs. 96.6%,P > 0.05)值。然而,在 BALF 分析中,AspLFD 的敏感性优于 GM 测定(100% vs. 88.9%),但差异无统计学意义(P > 0.05),特异性无差异(83.7% vs. 83.7%,P > 0.05)。在实体瘤队列中,AspLFD 和 GM 检测均表现出高灵敏度(均为 100%)和特异性(94.2% vs. 92.8%,P > 0.05)。
结论
AspLFD 在诊断癌症患者(尤其是实体瘤患者)的 IA 方面表现出良好的性能。因此,AspLFD 是一种替代 POCT,特别是当 GM 评估不易获得时。



























 京公网安备 11010802027423号
京公网安备 11010802027423号