Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Receptor for advanced glycation end products polymorphisms in coronary artery ectasia
Gene ( IF 3.5 ) Pub Date : 2024-04-07 , DOI: 10.1016/j.gene.2024.148450 Ezgi Irmak Aslan , Gulcin Ozkara , Onur Kilicarslan , Ozgur Selim Ser , Cem Bostan , Ahmet Yildiz , Ayca Diren Borekcioglu , Oguz Ozturk , Ozlem Kucukhuseyin , Hulya Yilmaz Aydogan
Gene ( IF 3.5 ) Pub Date : 2024-04-07 , DOI: 10.1016/j.gene.2024.148450 Ezgi Irmak Aslan , Gulcin Ozkara , Onur Kilicarslan , Ozgur Selim Ser , Cem Bostan , Ahmet Yildiz , Ayca Diren Borekcioglu , Oguz Ozturk , Ozlem Kucukhuseyin , Hulya Yilmaz Aydogan
|
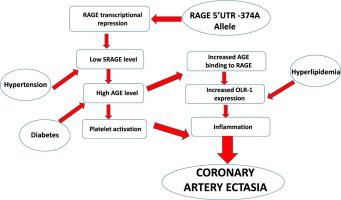
Although the implication of receptor of advanced glycation endproducts (RAGE) has been reported in coronary artery disease, its roles in coronary artery ectasia (CAE) have remained undetermined. Furthermore, the effect of polymorfisms were not well-defined in scope of soluble RAGE (sRAGE) levels. Thus, we aimed to investigate the influence of the functional polymorphisms of -374T > A (rs1800624) and G82S (rs2070600) in CAE development. This prospective observational study was conducted in 2 groups selected of 2452 patients who underwent elective coronary angiography (CAG) for evaluation after positive noninvasive heart tests. Group-I included 98 patients with non-obstructive coronary artery disease and CAE, and Group-II (control) included 100 patients with normal coronary arteries. SNPs were genotyped by real-time PCR using Taqman® genotyping assay. Serum sRAGE and soluble lectin-like oxidized receptor-1 (sOLR1) were assayed by ELISA and serum lipids were measured enzymatically. The frequencies of the -374A allele and -374AA genotype were significantly higher in CAE patients compared to controls (p < 0.001). sRAGE levels were not different between study groups, while sOLR1 levels were elevated in CAE (p = 0.004). In controls without systemic disease, -374A allele was associated with low sRAGE levels (p < 0.05), but this association was not significant in controls with HT. Similarly, sRAGE levels of CAE patients with both HT and T2DM were higher than those no systemic disease (p = 0.02). The -374A allele was also associated with younger patient age and higher platelet count in the CAE group in both total and subgroup analyses. In the correlation analyses, the -374A allele was also negatively correlated with age and positively correlated with Plt in all of these CAE groups. In the total CAE group, sRAGE levels also showed a positive correlation with age and a negative correlation with HDL-cholesterol levels. On the other hand, a negative correlation was observed between sRAGE and Plt in the total, hypertensive and no systemic disease control subgroups. Multivariate logistic regression analysis confirmed that the -374A allele (p < 0.001), hyperlipidemia (p < 0.05), and high sOLR1 level (p < 0.05) are risk factors for CAE. ROC curve analysis shows that RAGE -374A allele has AUC of 0.713 (sensitivity: 83.7 %, specificity: 59.0 %), which is higher than HLD (sensitivity: 59.2 %, specificity: 69.0 %), HT (sensitivity: 62.4 %, specificity: 61.1 %) and high sOLR1 level (≥0.67 ng/ml)) (sensitivity: 59.8 %, specificity: 58.5 %). Beside the demonstration of the relationship between -374A allele and increased risk of CAE for the first time, our results indicate that antihypertensive and antidiabetic treatment in CAE patients causes an increase in sRAGE levels. The lack of an association between the expected -374A allele and low sRAGE levels in total CAE group was attributed to the high proportion of hypertensive patients and hence to antihypertensive treatment. Moreover, the RAGE -374A allele is associated with younger age at CAE and higher Plt, suggesting that -374A may also be associated with platelet activation, which plays a role in the pathogenesis of CAE. However, our data need to be confirmed in a large study for definitive conclusions.
中文翻译:

冠状动脉扩张中晚期糖基化终末产物多态性受体
尽管已报道晚期糖基化终末产物受体(RAGE)在冠状动脉疾病中的作用,但其在冠状动脉扩张(CAE)中的作用仍未确定。此外,多态性对可溶性 RAGE (sRAGE) 水平范围的影响尚未明确。因此,我们的目的是研究-374T>A(rs1800624)和G82S(rs2070600)的功能多态性对CAE发展的影响。这项前瞻性观察性研究是在 2 组中进行的,从 2452 名患者中选出,这些患者在无创心脏检查呈阳性后接受了选择性冠状动脉造影 (CAG) 进行评估。第一组包括 98 名患有非阻塞性冠状动脉疾病和 CAE 的患者,第二组(对照组)包括 100 名冠状动脉正常的患者。使用 Taqman® 基因分型测定法通过实时 PCR 对 SNP 进行基因分型。通过 ELISA 测定血清 sRAGE 和可溶性凝集素样氧化受体 1 (sOLR1),并通过酶法测定血清脂质。与对照组相比,CAE 患者中 -374A 等位基因和 -374AA 基因型的频率显着较高(p < 0.001)。研究组之间的 sRAGE 水平没有差异,而 CAE 中 sOLR1 水平升高 (p = 0.004)。在没有全身性疾病的对照中,-374A 等位基因与低 sRAGE 水平相关 (p < 0.05),但这种关联在患有 HT 的对照中并不显着。同样,同时患有 HT 和 T2DM 的 CAE 患者的 sRAGE 水平高于没有全身性疾病的患者 (p = 0.02)。在总分析和亚组分析中,-374A 等位基因还与 CAE 组患者年龄较小和血小板计数较高相关。在相关性分析中,在所有这些 CAE 组中,-374A 等位基因也与年龄呈负相关,与 Plt 呈正相关。在总 CAE 组中,sRAGE 水平还显示与年龄呈正相关,与 HDL 胆固醇水平呈负相关。另一方面,在总亚组、高血压亚组和无全身疾病控制亚组中,sRAGE 和 Plt 之间观察到负相关。多变量逻辑回归分析证实,-374A 等位基因 (p < 0.001)、高脂血症 (p < 0.05) 和高 sOLR1 水平 (p < 0.05) 是 CAE 的危险因素。 ROC曲线分析显示,RAGE -374A等位基因的AUC为0.713(敏感性:83.7%,特异性:59.0%),高于HLD(敏感性:59.2%,特异性:69.0%)、HT(敏感性:62.4%,特异性) :61.1 %)和高 sOLR1 水平(≥0.67 ng/ml))(敏感性:59.8 %,特异性:58.5 %)。除了首次证明-374A等位基因与CAE风险增加之间的关系外,我们的结果表明CAE患者的抗高血压和抗糖尿病治疗会导致sRAGE水平增加。总 CAE 组中预期的 -374A 等位基因与低 sRAGE 水平之间缺乏关联,这归因于高血压患者比例较高,因此也与抗高血压治疗有关。此外,RAGE -374A 等位基因与 CAE 年龄较小和较高的 Plt 相关,表明 -374A 也可能与血小板活化有关,血小板活化在 CAE 的发病机制中发挥作用。然而,我们的数据需要在大型研究中得到证实才能得出明确的结论。
更新日期:2024-04-07
中文翻译:

冠状动脉扩张中晚期糖基化终末产物多态性受体
尽管已报道晚期糖基化终末产物受体(RAGE)在冠状动脉疾病中的作用,但其在冠状动脉扩张(CAE)中的作用仍未确定。此外,多态性对可溶性 RAGE (sRAGE) 水平范围的影响尚未明确。因此,我们的目的是研究-374T>A(rs1800624)和G82S(rs2070600)的功能多态性对CAE发展的影响。这项前瞻性观察性研究是在 2 组中进行的,从 2452 名患者中选出,这些患者在无创心脏检查呈阳性后接受了选择性冠状动脉造影 (CAG) 进行评估。第一组包括 98 名患有非阻塞性冠状动脉疾病和 CAE 的患者,第二组(对照组)包括 100 名冠状动脉正常的患者。使用 Taqman® 基因分型测定法通过实时 PCR 对 SNP 进行基因分型。通过 ELISA 测定血清 sRAGE 和可溶性凝集素样氧化受体 1 (sOLR1),并通过酶法测定血清脂质。与对照组相比,CAE 患者中 -374A 等位基因和 -374AA 基因型的频率显着较高(p < 0.001)。研究组之间的 sRAGE 水平没有差异,而 CAE 中 sOLR1 水平升高 (p = 0.004)。在没有全身性疾病的对照中,-374A 等位基因与低 sRAGE 水平相关 (p < 0.05),但这种关联在患有 HT 的对照中并不显着。同样,同时患有 HT 和 T2DM 的 CAE 患者的 sRAGE 水平高于没有全身性疾病的患者 (p = 0.02)。在总分析和亚组分析中,-374A 等位基因还与 CAE 组患者年龄较小和血小板计数较高相关。在相关性分析中,在所有这些 CAE 组中,-374A 等位基因也与年龄呈负相关,与 Plt 呈正相关。在总 CAE 组中,sRAGE 水平还显示与年龄呈正相关,与 HDL 胆固醇水平呈负相关。另一方面,在总亚组、高血压亚组和无全身疾病控制亚组中,sRAGE 和 Plt 之间观察到负相关。多变量逻辑回归分析证实,-374A 等位基因 (p < 0.001)、高脂血症 (p < 0.05) 和高 sOLR1 水平 (p < 0.05) 是 CAE 的危险因素。 ROC曲线分析显示,RAGE -374A等位基因的AUC为0.713(敏感性:83.7%,特异性:59.0%),高于HLD(敏感性:59.2%,特异性:69.0%)、HT(敏感性:62.4%,特异性) :61.1 %)和高 sOLR1 水平(≥0.67 ng/ml))(敏感性:59.8 %,特异性:58.5 %)。除了首次证明-374A等位基因与CAE风险增加之间的关系外,我们的结果表明CAE患者的抗高血压和抗糖尿病治疗会导致sRAGE水平增加。总 CAE 组中预期的 -374A 等位基因与低 sRAGE 水平之间缺乏关联,这归因于高血压患者比例较高,因此也与抗高血压治疗有关。此外,RAGE -374A 等位基因与 CAE 年龄较小和较高的 Plt 相关,表明 -374A 也可能与血小板活化有关,血小板活化在 CAE 的发病机制中发挥作用。然而,我们的数据需要在大型研究中得到证实才能得出明确的结论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号