当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxygen Electroreduction Reaction on Iron, Nitrogen-doped Nanocarbons: Structure – Reactivity Relationship
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.mcat.2024.114123 Anton V. Kuzmin , Bagrat A. Shainyan
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.mcat.2024.114123 Anton V. Kuzmin , Bagrat A. Shainyan
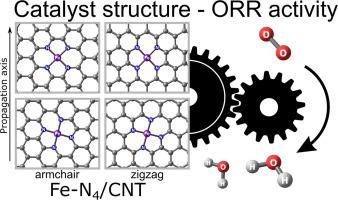
|
Theoretical calculations at the revPBE0-D3(PCM)/def2-TZVP level were performed to investigate the mechanism of the oxygen reduction reaction (ORR) on the FeN-doped NCMs (graphene, single-walled (6,6)-armchair and (12,0)-zigzag carbon nanotubes) as catalysts. The effect of the support and relative orientation of FeN fragment in the catalyst structure on the ORR activity and stability of the differently adsorbed successive intermediates (O*, HO*, O*, HO*, HO*, HO*) is discussed. Special attention is given to the possible poisoning effect of CO. The relative catalytic activity of the metal center and the adjacent C site of the carbon support are analyzed depending on the structure of the support. The metal catalytic center on the armchair nanotube and graphene supports is prone to deactivation by poisoning with CO, whereas in the zigzag nanotube supported catalyst the metal center is tolerant to CO. The four-electron mechanism of ORR is shown to be preferable over the two-electron mechanism in both acidic and alkaline media, both on the metal and C centers.
中文翻译:

铁、氮掺杂纳米碳上的氧电还原反应:结构-反应性关系
在 revPBE0-D3(PCM)/def2-TZVP 水平上进行理论计算,以研究 FeN 掺杂 NCM(石墨烯、单壁 (6,6)-扶手椅和( 12,0)-锯齿形碳纳米管)作为催化剂。讨论了催化剂结构中 FeN 片段的载体和相对方向对不同吸附的连续中间体 (O*、HO*、O*、HO*、HO*、HO*) 的 ORR 活性和稳定性的影响。特别注意CO可能的中毒效应。根据载体的结构分析金属中心和碳载体的相邻C位的相对催化活性。扶手椅纳米管和石墨烯载体上的金属催化中心容易因 CO 中毒而失活,而在锯齿形纳米管负载的催化剂中,金属中心对 CO 具有耐受性。ORR 的四电子机制被证明优于两种机制。 -酸性和碱性介质中金属和C中心上的电子机制。
更新日期:2024-04-15
中文翻译:

铁、氮掺杂纳米碳上的氧电还原反应:结构-反应性关系
在 revPBE0-D3(PCM)/def2-TZVP 水平上进行理论计算,以研究 FeN 掺杂 NCM(石墨烯、单壁 (6,6)-扶手椅和( 12,0)-锯齿形碳纳米管)作为催化剂。讨论了催化剂结构中 FeN 片段的载体和相对方向对不同吸附的连续中间体 (O*、HO*、O*、HO*、HO*、HO*) 的 ORR 活性和稳定性的影响。特别注意CO可能的中毒效应。根据载体的结构分析金属中心和碳载体的相邻C位的相对催化活性。扶手椅纳米管和石墨烯载体上的金属催化中心容易因 CO 中毒而失活,而在锯齿形纳米管负载的催化剂中,金属中心对 CO 具有耐受性。ORR 的四电子机制被证明优于两种机制。 -酸性和碱性介质中金属和C中心上的电子机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号