当前位置:
X-MOL 学术
›
Miner. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of pH and current density on the physical properties of cobalt obtained by electrowinning from sulfate solutions
Minerals Engineering ( IF 4.8 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.mineng.2024.108697 Fabiano Augusto Costa Mafra Passos , Jonas da Cruz Trajano de Souza , Iranildes Daniel dos Santos , Reiner Neumann , Pedro Paulo Medeiros Ribeiro , Achilles Junqueira Bourdot Dutra
Minerals Engineering ( IF 4.8 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.mineng.2024.108697 Fabiano Augusto Costa Mafra Passos , Jonas da Cruz Trajano de Souza , Iranildes Daniel dos Santos , Reiner Neumann , Pedro Paulo Medeiros Ribeiro , Achilles Junqueira Bourdot Dutra
|
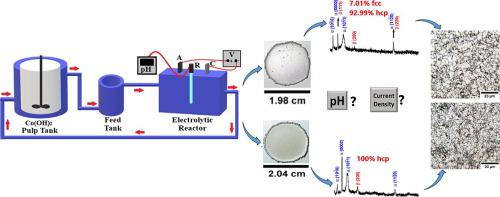
Cobalt is a critical metal, essential for the advancement of modern society, due to its applications on technology, energy and chemical industries. The primary aim of this paper was investigating, through voltammetric and bench scale electowinning tests from acidic cobalt sulfate solutions under typical industrial operational conditions, the effects of electrolyte pH and current density on parameters such as current efficiency (CE), specific energy consumption (SEC), morphology, structure and physical properties of the metallic cobalt deposit. The cobalt electrodeposits were characterized by X-ray diffraction (XRD), scanning electron microscopy coupled with energy dispersive spectrometry (SEM/EDS) and Vickers microhardness measurements. Results indicated that the deposition mechanisms, which are pH dependent, play an important role on the investigated parameters. A remarkable effect of both current density and electrolyte pH was observed. The highest CE, 95.5 %, was achieved with a current density of 400 A/m at pH 4.0, while the lowest SEC, 1.93 kWh/kg, was obtained with 200 A/m at pH3.0. Brighter and smoother deposits were obtained in tests at pH 5.0. The presence of the FCC-Co phase was only identified in the deposits obtained at pH 4.0, while at pH 3.0 and 5.0 only HCP-Co phase was observed, with differences in preferential crystallographic orientation. Tests with 400 A/m at pH 5.0 rendered the largest average crystallite and grain sizes (CS and GS, respectively), 68.8 nm and 33.0 µm respectively, whose microhardness (MH) was 169.8 HV, with a CE of 94.4 % and a SEC of 2.41 kWh/kg. The smallest CS and GS were obtained in the tests with current density of 400 A/m at pH 3.0, 11.9 nm and 1.8 µm respectively, rendering a MH value of 309.2 HV. Considering all the variables involved, the more attractive electrowinning conditions should be at pH 5.0 and 400 A/m, as they led to a smoother and more ductile deposit, despite the slightly higher SEC (2.41 kWh/kg).
中文翻译:

pH值和电流密度对硫酸盐溶液电积钴物理性质的影响
钴是一种关键金属,由于其在技术、能源和化学工业中的应用,对于现代社会的进步至关重要。本文的主要目的是通过在典型工业操作条件下对酸性硫酸钴溶液进行伏安法和小试规模的电解测试,研究电解液 pH 值和电流密度对电流效率 (CE)、比能量消耗 (SEC) 等参数的影响。 )、金属钴矿床的形态、结构和物理性质。通过 X 射线衍射 (XRD)、扫描电子显微镜结合能谱分析 (SEM/EDS) 和维氏显微硬度测量对钴电沉积物进行表征。结果表明,依赖于 pH 值的沉积机制对研究参数起着重要作用。观察到电流密度和电解质 pH 值的显着影响。在 pH 4.0 下,电流密度为 400 A/m 时,CE 最高,为 95.5%;在 pH 3.0 下,电流密度为 200 A/m 时,获得最低 SEC,1.93 kWh/kg。在 pH 5.0 的测试中获得了更明亮、更光滑的沉积物。仅在 pH 4.0 时获得的沉积物中发现了 FCC-Co 相的存在,而在 pH 3.0 和 5.0 时仅观察到 HCP-Co 相,但择优晶体取向存在差异。在 pH 5.0 下使用 400 A/m 进行测试,得到最大的平均微晶和晶粒尺寸(分别为 CS 和 GS),分别为 68.8 nm 和 33.0 µm,其显微硬度 (MH) 为 169.8 HV,CE 为 94.4 %,SEC 为2.41 千瓦时/公斤。在pH 3.0、电流密度为400 A/m、11.9 nm和1.8 µm的测试中获得最小的CS和GS,使MH值为309.2 HV。考虑到所有涉及的变量,更具吸引力的电解沉积条件应该是 pH 5.0 和 400 A/m,因为尽管 SEC 稍高(2.41 kWh/kg),但它们会产生更光滑、更具延展性的沉积物。
更新日期:2024-04-15
中文翻译:

pH值和电流密度对硫酸盐溶液电积钴物理性质的影响
钴是一种关键金属,由于其在技术、能源和化学工业中的应用,对于现代社会的进步至关重要。本文的主要目的是通过在典型工业操作条件下对酸性硫酸钴溶液进行伏安法和小试规模的电解测试,研究电解液 pH 值和电流密度对电流效率 (CE)、比能量消耗 (SEC) 等参数的影响。 )、金属钴矿床的形态、结构和物理性质。通过 X 射线衍射 (XRD)、扫描电子显微镜结合能谱分析 (SEM/EDS) 和维氏显微硬度测量对钴电沉积物进行表征。结果表明,依赖于 pH 值的沉积机制对研究参数起着重要作用。观察到电流密度和电解质 pH 值的显着影响。在 pH 4.0 下,电流密度为 400 A/m 时,CE 最高,为 95.5%;在 pH 3.0 下,电流密度为 200 A/m 时,获得最低 SEC,1.93 kWh/kg。在 pH 5.0 的测试中获得了更明亮、更光滑的沉积物。仅在 pH 4.0 时获得的沉积物中发现了 FCC-Co 相的存在,而在 pH 3.0 和 5.0 时仅观察到 HCP-Co 相,但择优晶体取向存在差异。在 pH 5.0 下使用 400 A/m 进行测试,得到最大的平均微晶和晶粒尺寸(分别为 CS 和 GS),分别为 68.8 nm 和 33.0 µm,其显微硬度 (MH) 为 169.8 HV,CE 为 94.4 %,SEC 为2.41 千瓦时/公斤。在pH 3.0、电流密度为400 A/m、11.9 nm和1.8 µm的测试中获得最小的CS和GS,使MH值为309.2 HV。考虑到所有涉及的变量,更具吸引力的电解沉积条件应该是 pH 5.0 和 400 A/m,因为尽管 SEC 稍高(2.41 kWh/kg),但它们会产生更光滑、更具延展性的沉积物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号