当前位置:
X-MOL 学术
›
Kidney Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A phase 2b, randomized, double-blind, placebo-controlled, clinical trial of atacicept for treatment of IgA nephropathy
Kidney International ( IF 19.6 ) Pub Date : 2024-03-27 , DOI: 10.1016/j.kint.2024.03.012 Richard Lafayette , Sean Barbour , Rubeen Israni , Xuelian Wei , Necmi Eren , Jürgen Floege , Vivekanand Jha , Sung Gyun Kim , Bart Maes , Richard K.S. Phoon , Harmeet Singh , Vladimír Tesař , Celia J.F. Lin , Jonathan Barratt
Kidney International ( IF 19.6 ) Pub Date : 2024-03-27 , DOI: 10.1016/j.kint.2024.03.012 Richard Lafayette , Sean Barbour , Rubeen Israni , Xuelian Wei , Necmi Eren , Jürgen Floege , Vivekanand Jha , Sung Gyun Kim , Bart Maes , Richard K.S. Phoon , Harmeet Singh , Vladimír Tesař , Celia J.F. Lin , Jonathan Barratt
|
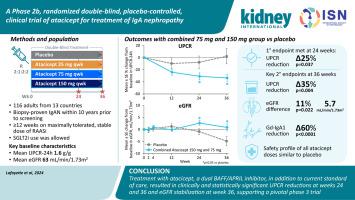
Atacicept is a first-in-class, dual anti-B-cell Activation Factor–A Proliferation-Inducing Ligand fusion protein in clinical evaluation for treatment of IgA nephropathy. To compare efficacy and safety of atacicept versus placebo in patients with IgAN, this randomized, double-blind, placebo-controlled phase 2b clinical trial ORIGIN enrolled 116 individuals with biopsy-proven IgA nephropathy. Participants were randomized to atacicept 150, 75, or 25 mg versus placebo once weekly for up to 36 weeks. Primary and key secondary endpoints were changes in urine protein creatinine ratio based on 24-hour urine collection at weeks 24 and 36, respectively, in the combined atacicept 150 mg and 75 mg group versus placebo. The primary endpoint was met at week 24 as the mean urine protein creatinine ratio was reduced from baseline by 31% in the combined atacicept group versus 8% with placebo, resulting in a significant 25% reduction with atacicept versus placebo. At week 36, the key secondary endpoint was met as the mean urine protein creatinine ratio reduced from baseline by 34% in the combined atacicept group versus a 2% increase with placebo, resulting in a significant 35% reduction with atacicept versus placebo. The reduction in proteinuria was accompanied by stabilization in endpoint eGFR with atacicept compared to a decline with placebo at week 36, resulting in significant between-group geometric mean difference of 11%, approximating an absolute difference of 5.7 mL/min/1.73m. Endpoint galactose deficient IgA1 levels significantly decreased from baseline by 60% versus placebo. The safety profile of atacicept was like placebo. Thus, our results provide evidence to support a pivotal, phase 3 study of atacicept in IgA nephropathy.
中文翻译:

阿西西普治疗 IgA 肾病的 2b 期、随机、双盲、安慰剂对照临床试验
Atacicept 是一种一流的双重抗 B 细胞激活因子 - 增殖诱导配体融合蛋白,用于 IgA 肾病治疗的临床评估。为了比较 atacicept 与安慰剂对 IgAN 患者的疗效和安全性,这项随机、双盲、安慰剂对照 2b 期临床试验 ORIGIN 招募了 116 名经活检证实患有 IgA 肾病的患者。参与者被随机分为每周一次接受 150、75 或 25 毫克阿塞西普与安慰剂组,持续长达 36 周。主要和关键次要终点是分别在第 24 周和第 36 周时基于 24 小时尿液收集的尿蛋白肌酐比值的变化,与安慰剂相比,联合阿塞西普 150 mg 和 75 mg 组。主要终点在第 24 周达到,联合阿塞西普组的平均尿蛋白肌酐比值较基线降低了 31%,而安慰剂组为 8%,与安慰剂组相比,阿塞西普组显着降低了 25%。第 36 周时,达到了关键的次要终点,阿西普联合治疗组的平均尿蛋白肌酐比值较基线降低了 34%,而安慰剂组则增加了 2%,因此与安慰剂相比,阿西普组的平均尿蛋白肌酐比值显着降低了 35%。第 36 周时,与安慰剂相比,蛋白尿减少的同时终点 eGFR 稳定,组间几何平均差异为 11%,绝对差异约为 5.7 mL/min/1.73m。与安慰剂相比,终点半乳糖缺乏 IgA1 水平较基线显着降低 60%。阿西西普的安全性与安慰剂相似。因此,我们的结果为支持阿西西普治疗 IgA 肾病的关键 3 期研究提供了证据。
更新日期:2024-03-27
中文翻译:

阿西西普治疗 IgA 肾病的 2b 期、随机、双盲、安慰剂对照临床试验
Atacicept 是一种一流的双重抗 B 细胞激活因子 - 增殖诱导配体融合蛋白,用于 IgA 肾病治疗的临床评估。为了比较 atacicept 与安慰剂对 IgAN 患者的疗效和安全性,这项随机、双盲、安慰剂对照 2b 期临床试验 ORIGIN 招募了 116 名经活检证实患有 IgA 肾病的患者。参与者被随机分为每周一次接受 150、75 或 25 毫克阿塞西普与安慰剂组,持续长达 36 周。主要和关键次要终点是分别在第 24 周和第 36 周时基于 24 小时尿液收集的尿蛋白肌酐比值的变化,与安慰剂相比,联合阿塞西普 150 mg 和 75 mg 组。主要终点在第 24 周达到,联合阿塞西普组的平均尿蛋白肌酐比值较基线降低了 31%,而安慰剂组为 8%,与安慰剂组相比,阿塞西普组显着降低了 25%。第 36 周时,达到了关键的次要终点,阿西普联合治疗组的平均尿蛋白肌酐比值较基线降低了 34%,而安慰剂组则增加了 2%,因此与安慰剂相比,阿西普组的平均尿蛋白肌酐比值显着降低了 35%。第 36 周时,与安慰剂相比,蛋白尿减少的同时终点 eGFR 稳定,组间几何平均差异为 11%,绝对差异约为 5.7 mL/min/1.73m。与安慰剂相比,终点半乳糖缺乏 IgA1 水平较基线显着降低 60%。阿西西普的安全性与安慰剂相似。因此,我们的结果为支持阿西西普治疗 IgA 肾病的关键 3 期研究提供了证据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号