Matter ( IF 18.9 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.matt.2024.03.010 Pei Li , Shuo Yang , Jiaxiong Zhu , Shengnan Wang , Yue Hou , Huilin Cui , Ze Chen , Rong Zhang , Zhuoxi Wu , Yiqiao Wang , Zhiquan Wei , Xinghui Liu , Shaoce Zhang , Xinliang Li , Chunyi Zhi
|
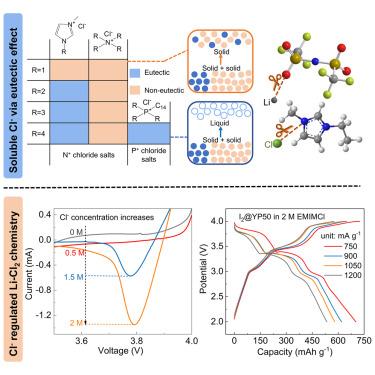
Chlorine-based electrochemical energy storage is a promising candidate for sustainable battery technology. The anionic redox reaction of Cl0/−1 is of interest due to its superior redox potential (1.36 V vs. standard hydrogen electrode [SHE]), capacity (756 mAh g−1), high power, and low cost. Although Cl chemistry has been used in aqueous batteries for a long time, its deployment in organic lithium batteries has been significantly impeded due to the insolubility of Cl− ions (<0.1 M). Scarce oxidizable Cl− blocks redox reactions and the inevitable lithium chloride (LiCl) film passivates electrodes on discharge. We report a eutectic effect to improve the Cl− solubility in organic electrolytes (2 M or higher) by mixing a series of N-/P-centered chloride salts with lithium bis(trifluoromethanesulfonyl)imide at specific ratios. Based on an optimized Cl− concentration, a Li-Cl2 cell using I as a chemical fixation can achieve a three-electron transfer with a specific capacity of 702 mAh g−1 and an energy density of 1,116 Wh kg−1.
中文翻译:

在高能量密度 Li-Cl2 电池的非水电解液中实现高浓度 Cl− 离子
氯基电化学储能是可持续电池技术的有前途的候选者。 Cl 的阴离子氧化还原反应因其优异的氧化还原电位(相对于标准氢电极 [SHE] 为 1.36 V)、容量(756 mAh g)、高功率和低成本而备受关注。尽管Cl化学在水系电池中的应用已有很长一段时间,但由于Cl离子的不溶性(<0.1 M),其在有机锂电池中的部署受到了严重阻碍。稀缺的可氧化 Cl 会阻碍氧化还原反应,而不可避免的氯化锂 (LiCl) 薄膜会在放电时钝化电极。我们报告了通过将一系列 N-/P-中心氯化物盐与双(三氟甲磺酰基)亚胺锂以特定比例混合来提高 Cl 在有机电解质(2 M 或更高)中的溶解度的共晶效应。基于优化的Cl浓度,使用I作为化学固定剂的Li-Cl电池可以实现三电子转移,比容量为702 mAh g,能量密度为1,116 Wh kg。



























 京公网安备 11010802027423号
京公网安备 11010802027423号