当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Study of the Esterase-like Activity of Albumin following Condensation by Macromolecular Crowding
Biomacromolecules ( IF 6.2 ) Pub Date : 2024-04-17 , DOI: 10.1021/acs.biomac.3c01431 Honorine Lamy 1 , Erika Bullier-Marchandin 1 , Cléo Pointel 1 , Aline Echalard 1 , Guy Daniel Ladam 1 , Gaëtan Lutzweiler 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2024-04-17 , DOI: 10.1021/acs.biomac.3c01431 Honorine Lamy 1 , Erika Bullier-Marchandin 1 , Cléo Pointel 1 , Aline Echalard 1 , Guy Daniel Ladam 1 , Gaëtan Lutzweiler 1
Affiliation
|
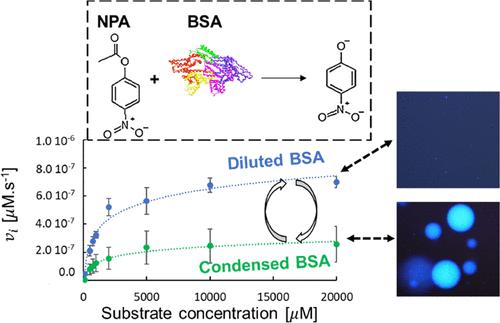
The ability of bovine serum albumin (BSA) to form condensates in crowded environments has been discovered only recently. Effects of this condensed state on the secondary structure of the protein have already been unraveled as some aging aspects, but the pseudo-enzymatic behavior of condensed BSA has never been reported yet. This article investigates the kinetic profile of para-nitrophenol acetate hydrolysis by BSA in its condensed state with poly(ethylene) glycol (PEG) as the crowding agent. Furthermore, the initial BSA concentration was varied between 0.25 and 1 mM which allowed us to modify the size distribution, the volume fraction, and the partition coefficient (varying from 136 to 180). Hence, the amount of BSA originally added was a simple way to modulate the size and density of the condensates. Compared with dilute BSA, the initial velocity (vi) with condensates was dramatically reduced. From the Michaelis–Menten fits, the extracted Michaelis constant Km and the maximum velocity Vmax decreased in control samples without condensates when the BSA concentration increased, which was attributed to BSA self-oligomerization. In samples containing condensates, the observed vi was interpreted as an effect of diluted BSA remaining in the supernatants and from the condensates. In supernatants, the crowding effect of PEG increased the kcat and catalytic efficiency. Last, Vmax was proportional to the volume fraction of the condensates, which could be controlled by varying its initial concentration. Hence, the major significance of this article is the control of the size and volume fraction of albumin condensates, along with their kinetic profile using liquid–liquid phase separation.
中文翻译:

大分子拥挤缩合后白蛋白类酯酶活性的动力学研究
牛血清白蛋白 (BSA) 在拥挤的环境中形成冷凝物的能力直到最近才被发现。这种缩合状态对蛋白质二级结构的影响已经被阐明为某些老化方面,但缩合 BSA 的伪酶行为尚未有报道。本文研究了以聚乙二醇 (PEG) 作为拥挤剂的缩合状态 BSA 水解对硝基苯酚乙酸酯的动力学曲线。此外,初始 BSA 浓度在 0.25 到 1 mM 之间变化,这使我们能够修改尺寸分布、体积分数和分配系数(从 136 到 180)。因此,最初添加的 BSA 量是调节冷凝物尺寸和密度的简单方法。与稀释的 BSA 相比,冷凝物的初速度 ( v i ) 显着降低。根据 Michaelis-Menten 拟合,当 BSA 浓度增加时,在没有冷凝物的对照样品中,提取的米氏常数K m和最大速度V max降低,这归因于 BSA 自低聚。在含有冷凝物的样品中,观察到的vi被解释为残留在上清液和冷凝物中的稀释 BSA 的影响。在上清液中,PEG 的拥挤效应增加了k cat和催化效率。最后,V max与冷凝物的体积分数成正比,这可以通过改变其初始浓度来控制。因此,本文的主要意义是利用液-液相分离控制白蛋白冷凝物的尺寸和体积分数,以及它们的动力学曲线。
更新日期:2024-04-18
中文翻译:

大分子拥挤缩合后白蛋白类酯酶活性的动力学研究
牛血清白蛋白 (BSA) 在拥挤的环境中形成冷凝物的能力直到最近才被发现。这种缩合状态对蛋白质二级结构的影响已经被阐明为某些老化方面,但缩合 BSA 的伪酶行为尚未有报道。本文研究了以聚乙二醇 (PEG) 作为拥挤剂的缩合状态 BSA 水解对硝基苯酚乙酸酯的动力学曲线。此外,初始 BSA 浓度在 0.25 到 1 mM 之间变化,这使我们能够修改尺寸分布、体积分数和分配系数(从 136 到 180)。因此,最初添加的 BSA 量是调节冷凝物尺寸和密度的简单方法。与稀释的 BSA 相比,冷凝物的初速度 ( v i ) 显着降低。根据 Michaelis-Menten 拟合,当 BSA 浓度增加时,在没有冷凝物的对照样品中,提取的米氏常数K m和最大速度V max降低,这归因于 BSA 自低聚。在含有冷凝物的样品中,观察到的vi被解释为残留在上清液和冷凝物中的稀释 BSA 的影响。在上清液中,PEG 的拥挤效应增加了k cat和催化效率。最后,V max与冷凝物的体积分数成正比,这可以通过改变其初始浓度来控制。因此,本文的主要意义是利用液-液相分离控制白蛋白冷凝物的尺寸和体积分数,以及它们的动力学曲线。



























 京公网安备 11010802027423号
京公网安备 11010802027423号