当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alcohols as Substrates in Transition-Metal-Catalyzed Arylation, Alkylation, and Related Reactions
Chemical Reviews ( IF 62.1 ) Pub Date : 2024-04-17 , DOI: 10.1021/acs.chemrev.4c00094 Adam Cook 1 , Stephen G. Newman 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2024-04-17 , DOI: 10.1021/acs.chemrev.4c00094 Adam Cook 1 , Stephen G. Newman 1
Affiliation
|
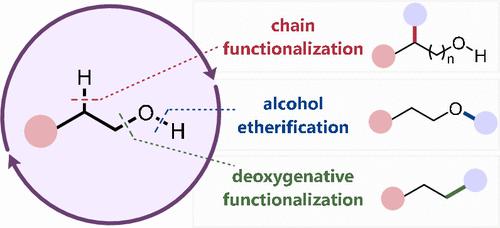
Alcohols are abundant and attractive feedstock molecules for organic synthesis. Many methods for their functionalization require them to first be converted into a more activated derivative, while recent years have seen a vast increase in the number of complexity-building transformations that directly harness unprotected alcohols. This Review discusses how transition metal catalysis can be used toward this goal. These transformations are broadly classified into three categories. Deoxygenative functionalizations, representing derivatization of the C–O bond, enable the alcohol to act as a leaving group toward the formation of new C–C bonds. Etherifications, characterized by derivatization of the O–H bond, represent classical reactivity that has been modernized to include mild reaction conditions, diverse reaction partners, and high selectivities. Lastly, chain functionalization reactions are described, wherein the alcohol group acts as a mediator in formal C–H functionalization reactions of the alkyl backbone. Each of these three classes of transformation will be discussed in context of intermolecular arylation, alkylation, and related reactions, illustrating how catalysis can enable alcohols to be directly harnessed for organic synthesis.
中文翻译:

过渡金属催化芳基化、烷基化及相关反应中醇作为底物
醇是有机合成中丰富且有吸引力的原料分子。许多功能化方法要求它们首先转化为更活化的衍生物,而近年来直接利用未受保护的醇的复杂性构建转化的数量大幅增加。本综述讨论了如何利用过渡金属催化来实现这一目标。这些转变大致分为三类。脱氧官能化代表 C-O 键的衍生化,使醇能够充当离去基团,从而形成新的 C-C 键。醚化反应的特点是 O-H 键的衍生化,代表了经典的反应性,该反应性已被现代化,包括温和的反应条件、多样化的反应伙伴和高选择性。最后,描述了链官能化反应,其中醇基团在烷基主链的正式 C-H 官能化反应中充当介体。这三类转化中的每一类都将在分子间芳基化、烷基化和相关反应的背景下进行讨论,说明催化如何使醇能够直接用于有机合成。
更新日期:2024-04-18
中文翻译:

过渡金属催化芳基化、烷基化及相关反应中醇作为底物
醇是有机合成中丰富且有吸引力的原料分子。许多功能化方法要求它们首先转化为更活化的衍生物,而近年来直接利用未受保护的醇的复杂性构建转化的数量大幅增加。本综述讨论了如何利用过渡金属催化来实现这一目标。这些转变大致分为三类。脱氧官能化代表 C-O 键的衍生化,使醇能够充当离去基团,从而形成新的 C-C 键。醚化反应的特点是 O-H 键的衍生化,代表了经典的反应性,该反应性已被现代化,包括温和的反应条件、多样化的反应伙伴和高选择性。最后,描述了链官能化反应,其中醇基团在烷基主链的正式 C-H 官能化反应中充当介体。这三类转化中的每一类都将在分子间芳基化、烷基化和相关反应的背景下进行讨论,说明催化如何使醇能够直接用于有机合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号