Cellular and Molecular Neurobiology ( IF 4 ) Pub Date : 2024-04-17 , DOI: 10.1007/s10571-024-01467-4 Qi Yun , Si-Fei Ma , Wei-Ning Zhang , Meng Gu , Jia Wang
|
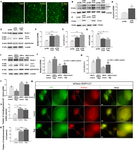
An increasing body of research suggests that promoting microglial autophagy hinders the neuroinflammation initiated though the NLRP3 inflammasome activation in Alzheimer’s disease (AD). The function of FoxG1, a crucial transcription factor involved in cell survival by regulating mitochondrial function, remains unknown during the AD process and neuroinflammation occurs. In the present study, we firstly found that Aβ peptides induced AD-like neuroinflammation upregulation and downregulated the level of autophagy. Following low-dose Aβ25–35 stimulation, FoxG1 expression and autophagy exhibited a gradual increase. Nevertheless, with high-concentration Aβ25–35 treatment, progressive decrease in FoxG1 expression and autophagy levels as the concentration of Aβ25–35 escalated. In addition, FoxG1 has a positive effect on cell viability and autophagy in the nervous system. In parallel with the Aβ25–35 stimulation, we employed siRNA to decrease the expression of FoxG1 in N2A cells. A substantial reduction in autophagy level (Beclin1, LC3II, SQSTM1/P62) and a notable growth in inflammatory response (NLRP3, TNF-α, and IL-6) were observed. In addition, we found FoxG1 overexpression owned the effect on the activation of AMPK/mTOR autophagy pathway and siRNA-FoxG1 successfully abolished this effect. Lastly, FoxG1 suppressed the NLRP3 inflammasome and enhanced the cognitive function in AD-like mouse model induced by Aβ25–35. Confirmed by cellular and animal experiments, FoxG1 suppressed NLRP3-mediated neuroinflammation, which was strongly linked to autophagy regulated by AMPK/mTOR. Taken together, FoxG1 may be a critical node in the pathologic progression of AD and has the potential to serve as therapeutic target.
中文翻译:

FoxG1 作为阿尔茨海默病的潜在治疗靶点:通过 AMPK/mTOR 自噬途径调节 NLRP3 炎症小体
越来越多的研究表明,促进小胶质细胞自噬会阻碍阿尔茨海默病 (AD) 中 NLRP3 炎症小体激活引发的神经炎症。 FoxG1 是一种通过调节线粒体功能参与细胞生存的关键转录因子,但在 AD 过程和神经炎症发生过程中其功能仍然未知。在本研究中,我们首次发现Aβ肽可诱导AD样神经炎症上调并下调自噬水平。低剂量 Aβ25-35 刺激后,FoxG1 表达和自噬逐渐增加。然而,在高浓度 Aβ25-35 处理下,随着 Aβ25-35 浓度的升高,FoxG1 表达和自噬水平逐渐降低。此外,FoxG1 对神经系统中的细胞活力和自噬具有积极作用。在 Aβ25-35 刺激的同时,我们使用 siRNA 来降低 N2A 细胞中 FoxG1 的表达。观察到自噬水平(Beclin1、LC3II、SQSTM1/P62)显着降低,炎症反应(NLRP3、TNF-α 和 IL-6)显着增加。此外,我们发现FoxG1过表达对AMPK/mTOR自噬通路的激活有影响,而siRNA-FoxG1成功消除了这种影响。最后,在 Aβ25-35 诱导的 AD 样小鼠模型中,FoxG1 抑制 NLRP3 炎性体并增强认知功能。经细胞和动物实验证实,FoxG1 抑制 NLRP3 介导的神经炎症,这与 AMPK/mTOR 调节的自噬密切相关。综上所述,FoxG1可能是AD病理进展的关键节点,并有可能作为治疗靶点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号