当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methanetriol─Formation of an Impossible Molecule
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.4c02637 Joshua H. Marks 1, 2 , Xilin Bai 3 , Anatoliy A. Nikolayev 4 , Qi’ang Gong 3 , Cheng Zhu 1, 2 , N. Fabian Kleimeier 1, 2 , Andrew M. Turner 1, 2 , Santosh K. Singh 1, 2 , Jia Wang 1, 2 , Jiuzhong Yang 5 , Yang Pan 5 , Tao Yang 3, 6 , Alexander M. Mebel 7 , Ralf I. Kaiser 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.4c02637 Joshua H. Marks 1, 2 , Xilin Bai 3 , Anatoliy A. Nikolayev 4 , Qi’ang Gong 3 , Cheng Zhu 1, 2 , N. Fabian Kleimeier 1, 2 , Andrew M. Turner 1, 2 , Santosh K. Singh 1, 2 , Jia Wang 1, 2 , Jiuzhong Yang 5 , Yang Pan 5 , Tao Yang 3, 6 , Alexander M. Mebel 7 , Ralf I. Kaiser 1, 2
Affiliation
|
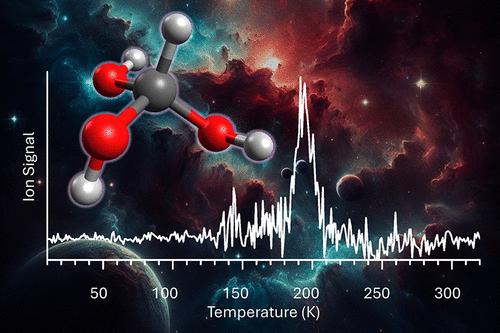
Orthocarboxylic acids─organic molecules carrying three hydroxyl groups at the same carbon atom─have been distinguished as vital reactive intermediates by the atmospheric science and physical (organic) chemistry communities as transients in the atmospheric aerosol cycle. Predicted short lifetimes and their tendency to dehydrate to a carboxylic acid, free orthocarboxylic acids, signify one of the most elusive classes of organic reactive intermediates, with even the simplest representative methanetriol (CH(OH)3)─historically known as orthoformic acid─not previously been detected experimentally. Here, we report the first synthesis of the previously elusive methanetriol molecule in low-temperature mixed methanol (CH3OH) and molecular oxygen (O2) ices subjected to energetic irradiation. Supported by electronic structure calculations, methanetriol was identified in the gas phase upon sublimation via isomer-selective photoionization reflectron time-of-flight mass spectrometry combined with isotopic substitution studies and the detection of photoionization fragments. The first synthesis and detection of methanetriol (CH(OH)3) reveals its gas-phase stability as supported by a significant barrier hindering unimolecular decomposition. These findings progress our fundamental understanding of the chemistry and chemical bonding of methanetriol, hydroxyperoxymethane (CH3OOOH), and hydroxyperoxymethanol (CH2(OH)OOH), which are all prototype molecules in the oxidation chemistry of the atmosphere.
中文翻译:

甲三醇─不可能分子的形成
原羧酸(在同一碳原子上带有三个羟基的有机分子)已被大气科学和物理(有机)化学界视为重要的反应中间体,作为大气气溶胶循环中的瞬态物质。预计的较短寿命及其脱水生成羧酸(游离原羧酸)的倾向,标志着最难以捉摸的一类有机反应中间体,即使是最简单的代表性甲三醇 (CH(OH) 3) — 历史上称为原甲酸 — 也不是之前已经通过实验检测到。在这里,我们报告了在低温混合甲醇(CH 3 OH)和分子氧(O 2)冰中经受高能辐射首次合成了以前难以捉摸的甲三醇分子。在电子结构计算的支持下,通过异构体选择性光电离反射飞行时间质谱结合同位素替代研究和光电离碎片检测,在升华时鉴定出气相中的甲烷三醇。甲三醇 (CH(OH) 3 )的首次合成和检测揭示了其气相稳定性,这得益于阻碍单分子分解的重要屏障。这些发现增进了我们对甲三醇、羟基过氧甲烷 (CH 3 OOOH) 和羟基过氧甲醇 (CH 2 (OH)OOH)的化学和化学键的基本理解,这些都是大气氧化化学中的原型分子。
更新日期:2024-04-17
中文翻译:

甲三醇─不可能分子的形成
原羧酸(在同一碳原子上带有三个羟基的有机分子)已被大气科学和物理(有机)化学界视为重要的反应中间体,作为大气气溶胶循环中的瞬态物质。预计的较短寿命及其脱水生成羧酸(游离原羧酸)的倾向,标志着最难以捉摸的一类有机反应中间体,即使是最简单的代表性甲三醇 (CH(OH) 3) — 历史上称为原甲酸 — 也不是之前已经通过实验检测到。在这里,我们报告了在低温混合甲醇(CH 3 OH)和分子氧(O 2)冰中经受高能辐射首次合成了以前难以捉摸的甲三醇分子。在电子结构计算的支持下,通过异构体选择性光电离反射飞行时间质谱结合同位素替代研究和光电离碎片检测,在升华时鉴定出气相中的甲烷三醇。甲三醇 (CH(OH) 3 )的首次合成和检测揭示了其气相稳定性,这得益于阻碍单分子分解的重要屏障。这些发现增进了我们对甲三醇、羟基过氧甲烷 (CH 3 OOOH) 和羟基过氧甲醇 (CH 2 (OH)OOH)的化学和化学键的基本理解,这些都是大气氧化化学中的原型分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号