当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of the Squaramide Scaffold for High Potential and Multielectron Catholytes for Use in Redox Flow Batteries
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.3c14776 Jacob S. Tracy 1, 2, 3, 4 , Conor H. Broderick 1, 2, 3 , F. Dean Toste 1, 2, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.3c14776 Jacob S. Tracy 1, 2, 3, 4 , Conor H. Broderick 1, 2, 3 , F. Dean Toste 1, 2, 3
Affiliation
|
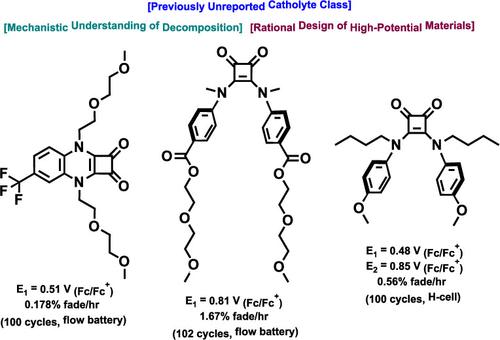
Nonaqueous organic redox flow batteries (N-ORFBs) are a promising technology for grid-scale storage of energy generated from intermittent renewable sources. Their primary benefit over traditional aqueous RFBs is the wide electrochemical stability window of organic solvents, but the design of catholyte materials, which can exploit the upper range of this window, has proven challenging. We report herein a new class of N-ORFB catholytes in the form of squaric acid quinoxaline (SQX) and squaric acid amide (SQA) materials. Mechanistic investigation of decomposition in battery-relevant conditions via NMR, HRMS, and electrochemical methods enabled a rational design approach to optimizing these scaffolds. Three lead compounds were developed: a highly stable one-electron SQX material with an oxidation potential of 0.51 V vs Fc/Fc+ that maintained 99% of peak capacity after 102 cycles (51 h) when incorporated into a 1.58 V flow battery; a high-potential one-electron SQA material with an oxidation potential of 0.81 V vs Fc/Fc+ that demonstrated negligible loss of redox active material as measured by pre- and postcycling CV peak currents when incorporated in a 1.63 V flow battery for 110 cycles over 29 h; and a proof-of-concept two-electron SQA catholyte material with oxidation potentials of 0.48 and 0.85 V vs Fc/Fc+ that demonstrated a capacity fade of just 0.56% per hour during static H-cell cycling. These findings expand the previously reported space of high-potential catholyte materials and showcase the power of mechanistically informed synthetic design for N-ORFB materials development.
中文翻译:

用于氧化还原液流电池的高电位和多电子阴极电解液的方酰胺支架的开发
非水有机氧化还原液流电池(N-ORFB)是一种很有前途的技术,用于电网规模存储间歇性可再生能源产生的能源。与传统水性 RFB 相比,它们的主要优点是有机溶剂具有较宽的电化学稳定性窗口,但事实证明,能够利用该窗口上限的阴极电解液材料的设计具有挑战性。我们在此报告了一类新型 N-ORFB 阴极电解质,其形式为方酸喹喔啉 (SQX) 和方酸酰胺 (SQA) 材料。通过 NMR、HRMS 和电化学方法对电池相关条件下的分解进行机理研究,为优化这些支架提供了合理的设计方法。开发了三种先导化合物:一种高度稳定的单电子 SQX 材料,其氧化电位相对于 Fc/Fc +为 0.51 V ,当并入 1.58 V 液流电池时,在 102 个循环(51 小时)后仍保持 99% 的峰值容量;一种高电位单电子 SQA 材料,其氧化电位相对于 Fc/Fc +为 0.81 V ,当并入 1.63 V 液流电池 110 个循环时,通过循环前后 CV 峰值电流测量,氧化还原活性材料的损失可以忽略不计超过29小时;概念验证的双电子 SQA 阴极电解液材料的氧化电位相对于 Fc/Fc +为 0.48 和 0.85 V ,在静态 H 电池循环过程中每小时容量衰减仅为 0.56%。这些发现扩展了先前报道的高潜力阴极电解液材料的空间,并展示了 N-ORFB 材料开发的机械通知合成设计的力量。
更新日期:2024-04-17
中文翻译:

用于氧化还原液流电池的高电位和多电子阴极电解液的方酰胺支架的开发
非水有机氧化还原液流电池(N-ORFB)是一种很有前途的技术,用于电网规模存储间歇性可再生能源产生的能源。与传统水性 RFB 相比,它们的主要优点是有机溶剂具有较宽的电化学稳定性窗口,但事实证明,能够利用该窗口上限的阴极电解液材料的设计具有挑战性。我们在此报告了一类新型 N-ORFB 阴极电解质,其形式为方酸喹喔啉 (SQX) 和方酸酰胺 (SQA) 材料。通过 NMR、HRMS 和电化学方法对电池相关条件下的分解进行机理研究,为优化这些支架提供了合理的设计方法。开发了三种先导化合物:一种高度稳定的单电子 SQX 材料,其氧化电位相对于 Fc/Fc +为 0.51 V ,当并入 1.58 V 液流电池时,在 102 个循环(51 小时)后仍保持 99% 的峰值容量;一种高电位单电子 SQA 材料,其氧化电位相对于 Fc/Fc +为 0.81 V ,当并入 1.63 V 液流电池 110 个循环时,通过循环前后 CV 峰值电流测量,氧化还原活性材料的损失可以忽略不计超过29小时;概念验证的双电子 SQA 阴极电解液材料的氧化电位相对于 Fc/Fc +为 0.48 和 0.85 V ,在静态 H 电池循环过程中每小时容量衰减仅为 0.56%。这些发现扩展了先前报道的高潜力阴极电解液材料的空间,并展示了 N-ORFB 材料开发的机械通知合成设计的力量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号